NDC Code(s) : 58411-272-60, 58411-273-60, 58411-274-60, 58411-275-60, 58411-276-60, 58411-277-60, 58411-278-60, 58411-279-60, 58411-280-60, 58411-281-60, 58411-282-60, 58411-283-60
Packager : SHISEIDO AMERICAS CORPORATION
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - SHISEIDO AMERICAS CORPORATION(193691821) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| SHISEIDO AMERICA INC. | 782677132 | manufacture(58411-272, 58411-273, 58411-274, 58411-275, 58411-276, 58411-277, 58411-278, 58411-279, 58411-280, 58411-281, 58411-282, 58411-283), analysis(58411-272, 58411-273, 58411-274, 58411-275, 58411-276, 58411-277, 58411-278, 58411-279, 58411-280, 58411-281, 58411-282, 58411-283) | |
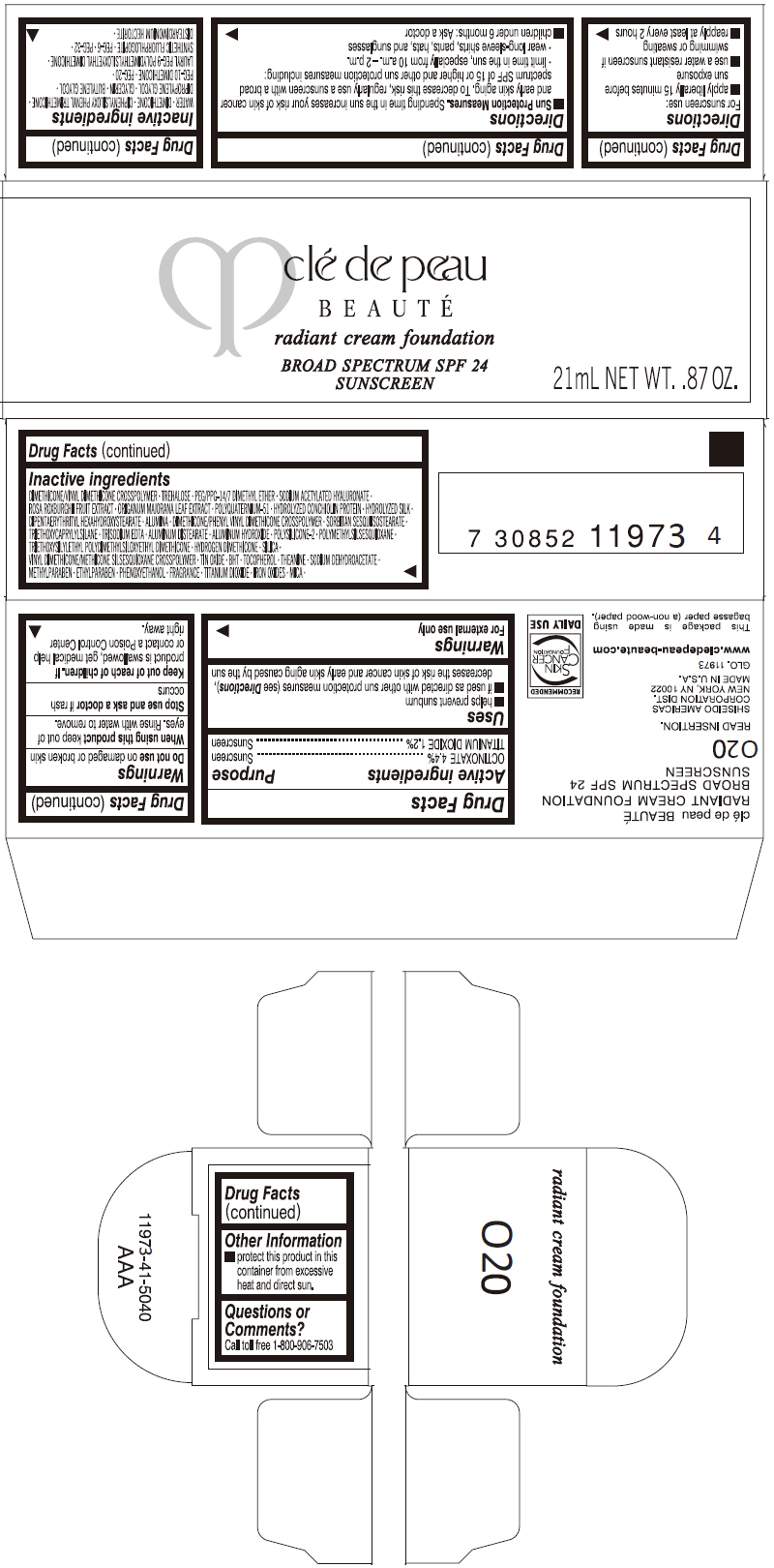
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
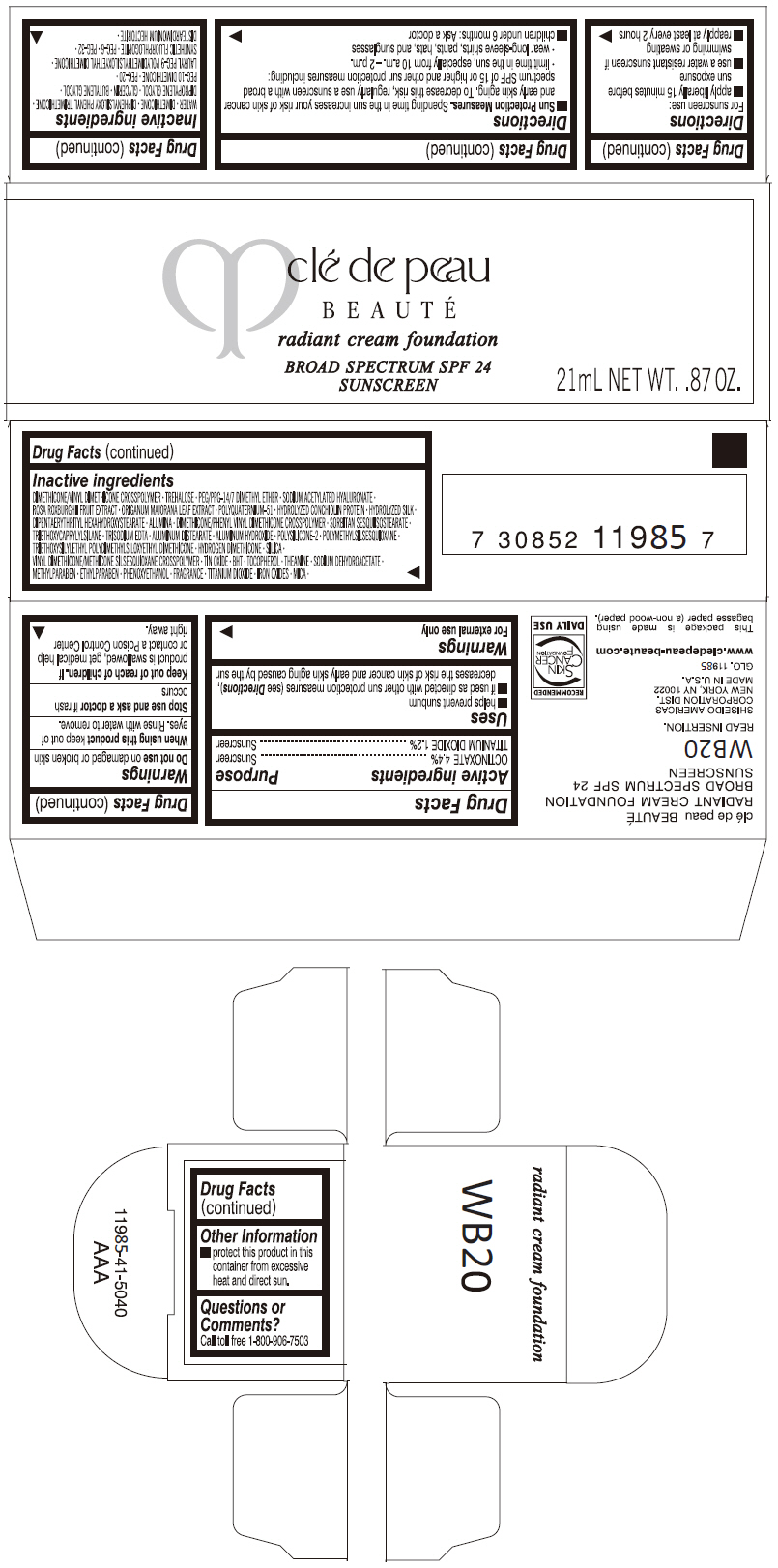
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
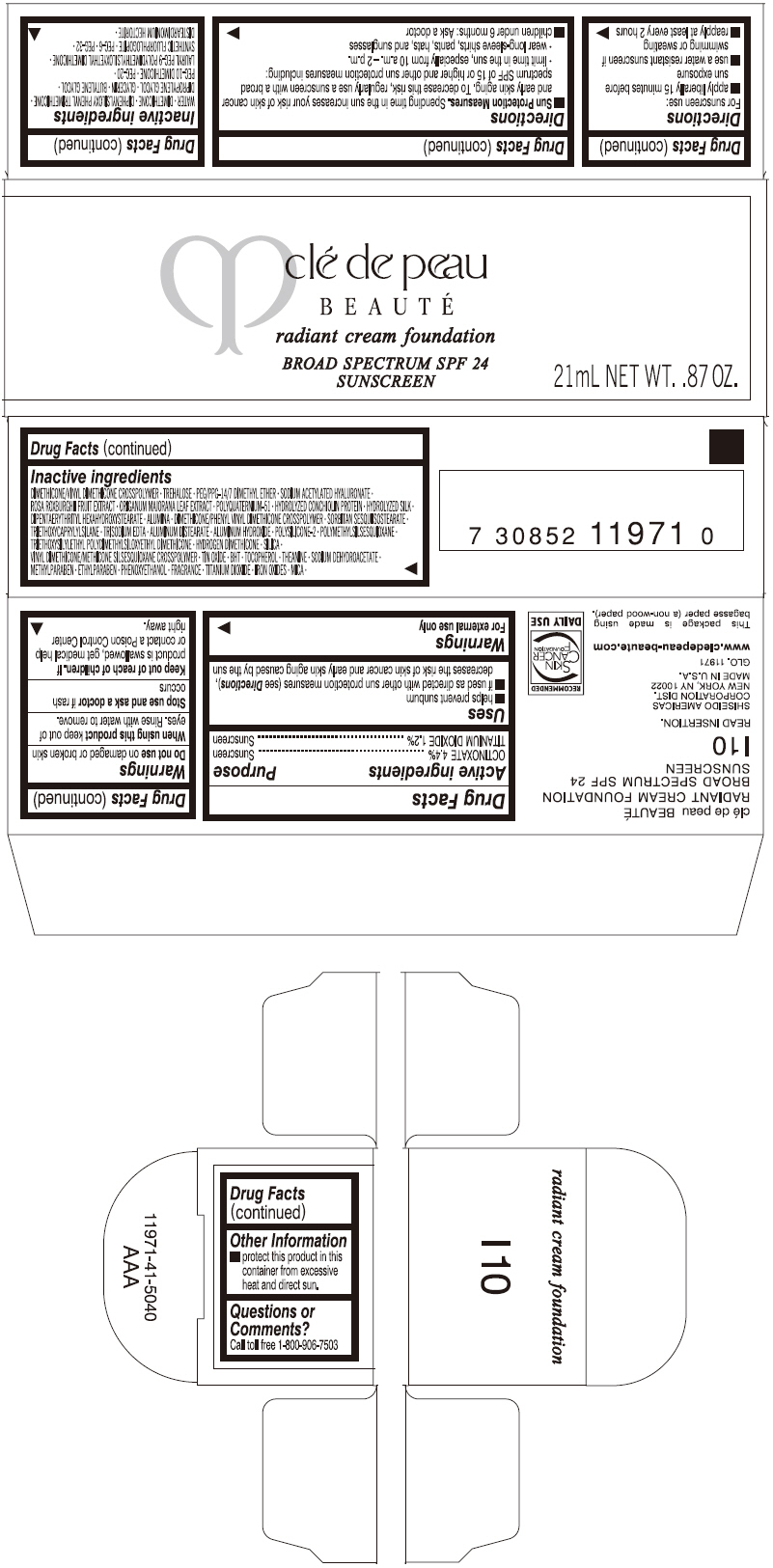
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
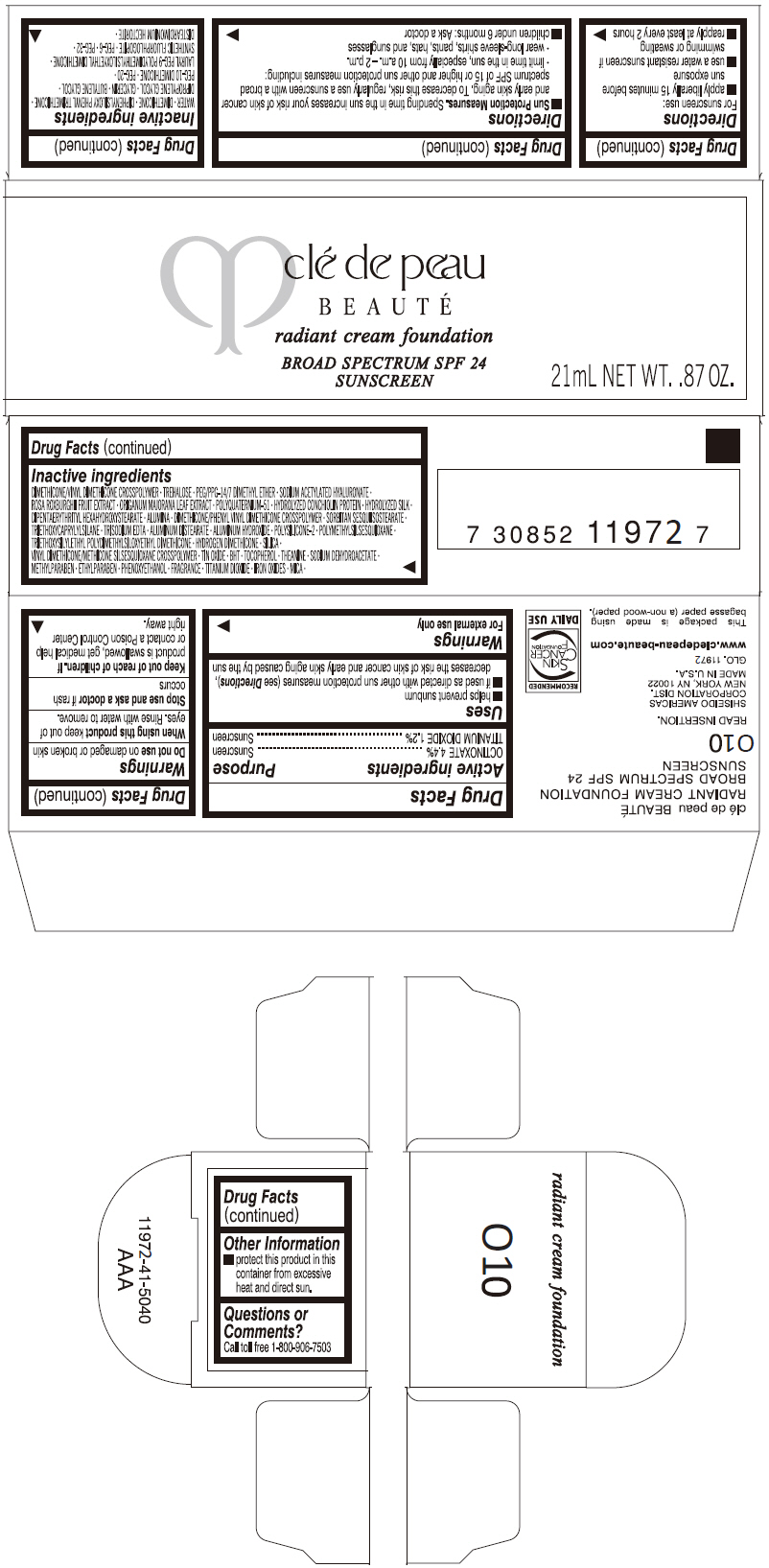
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
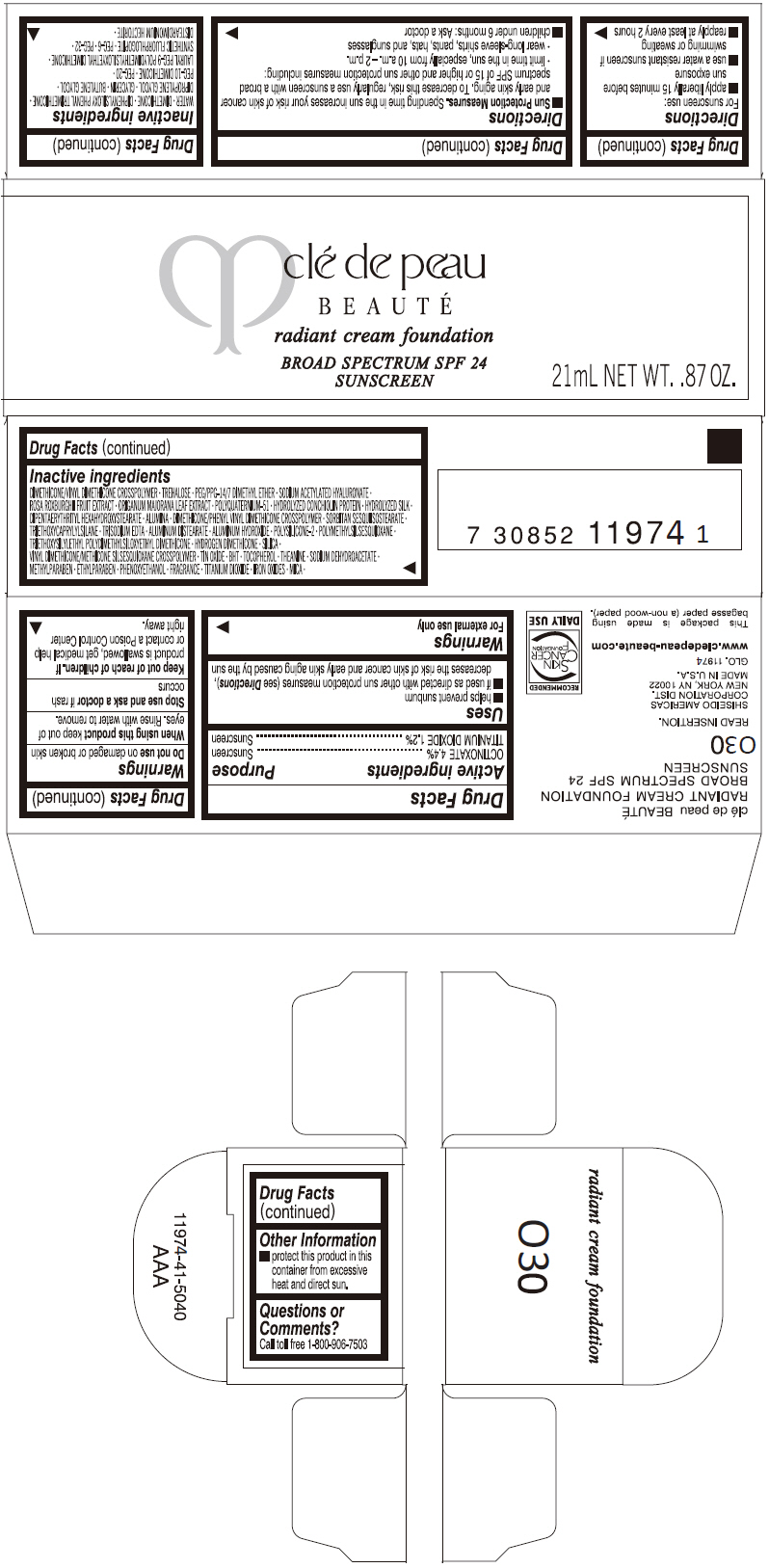
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
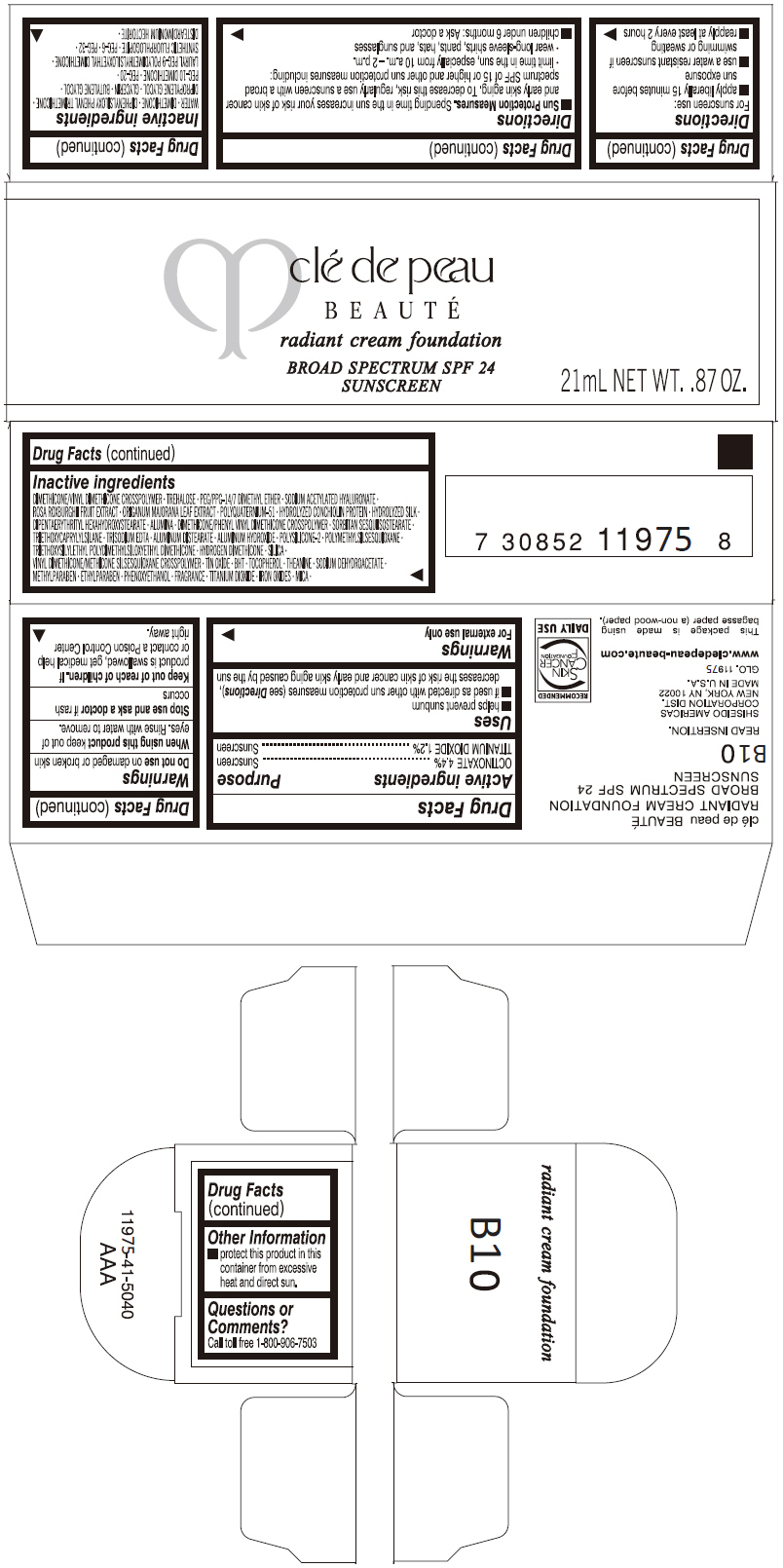
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
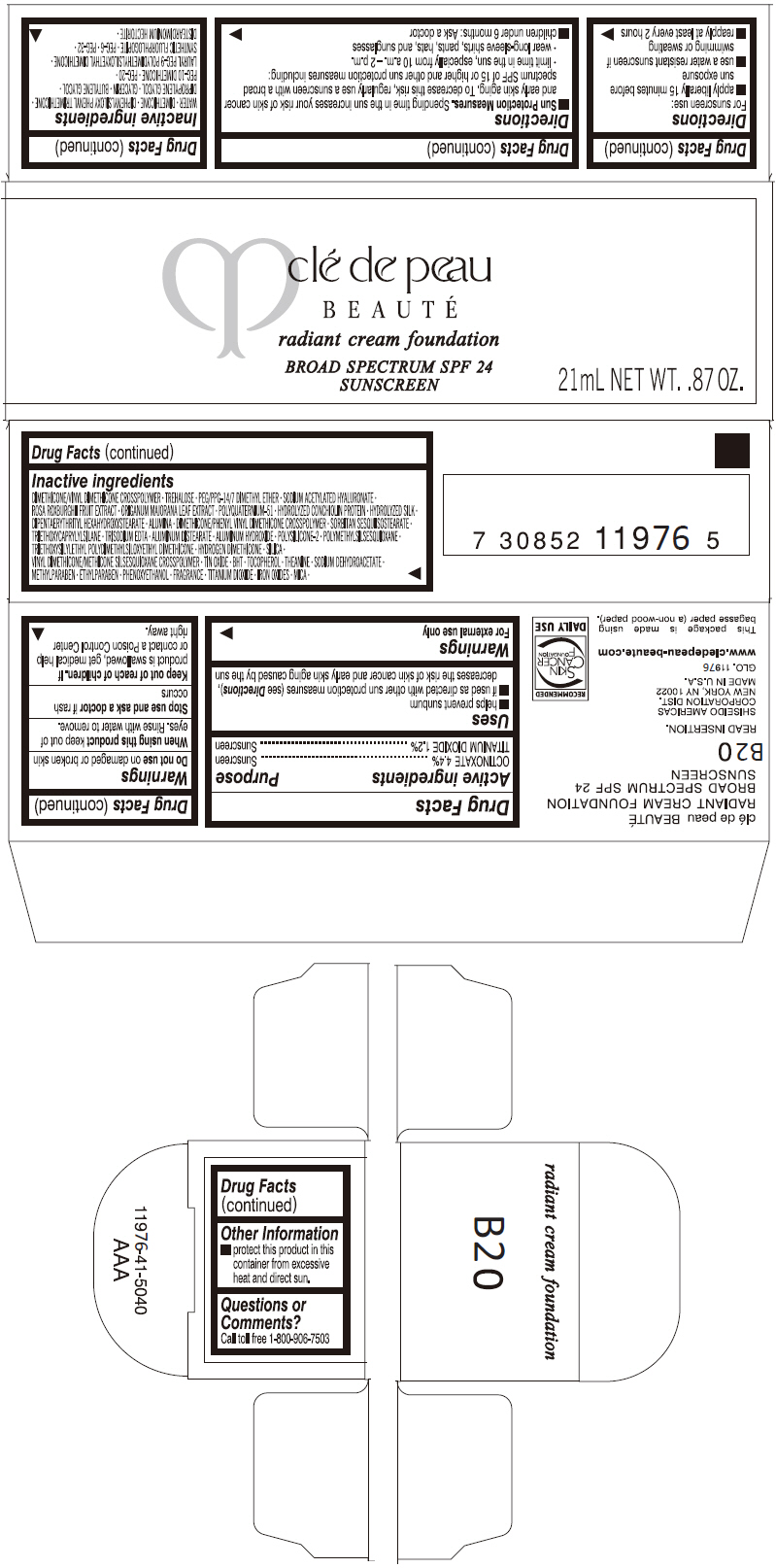
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
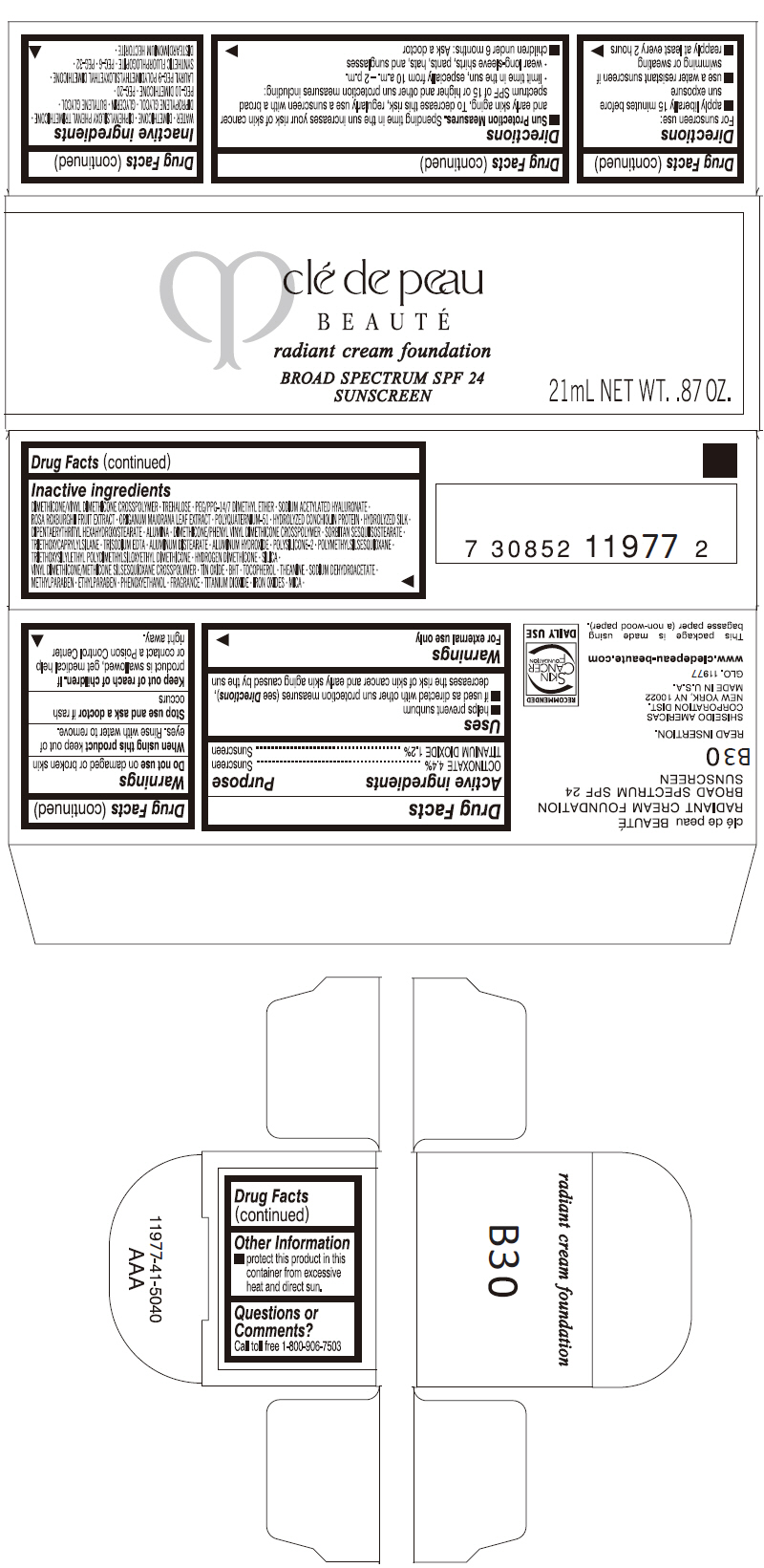
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
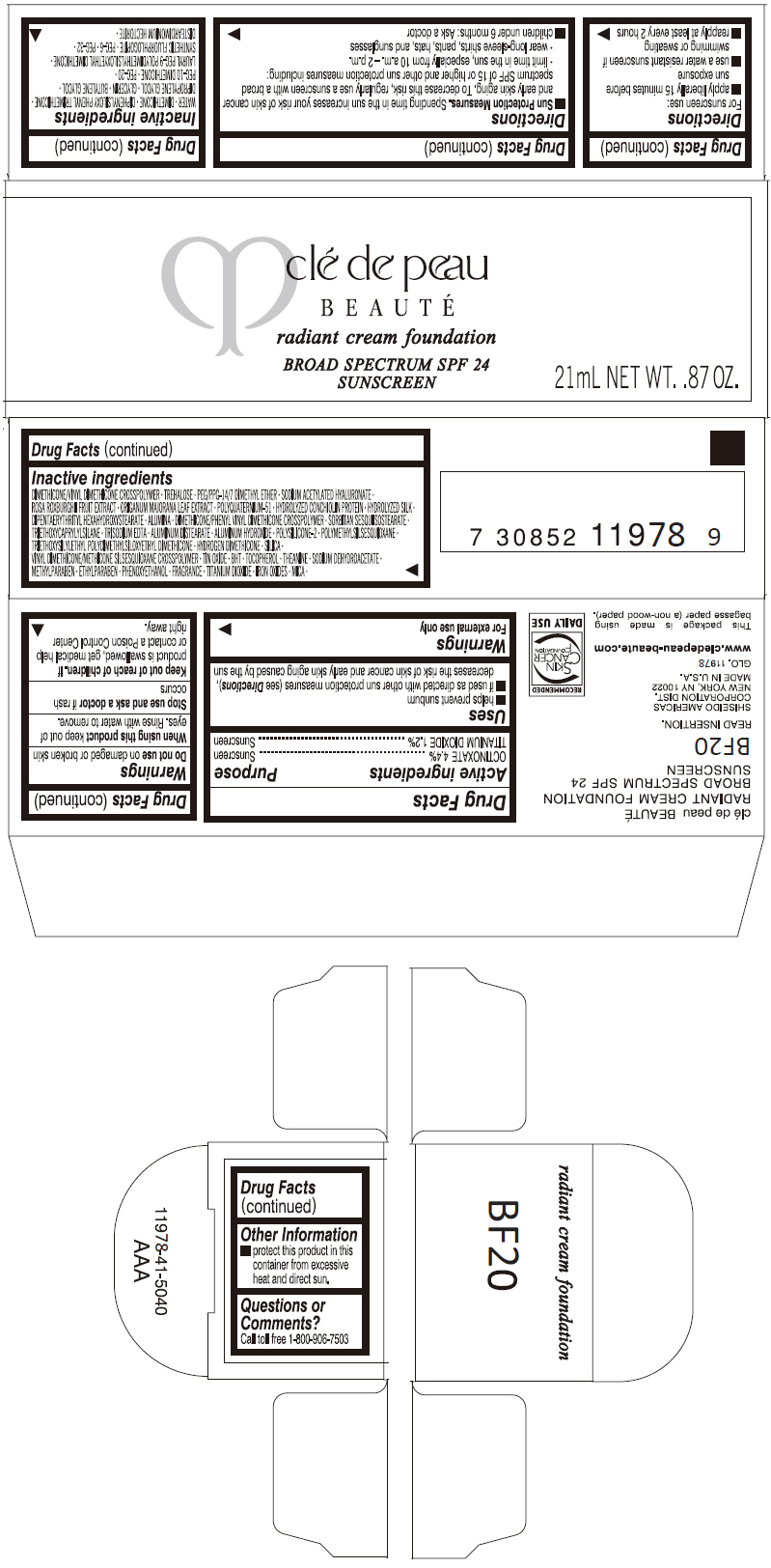
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
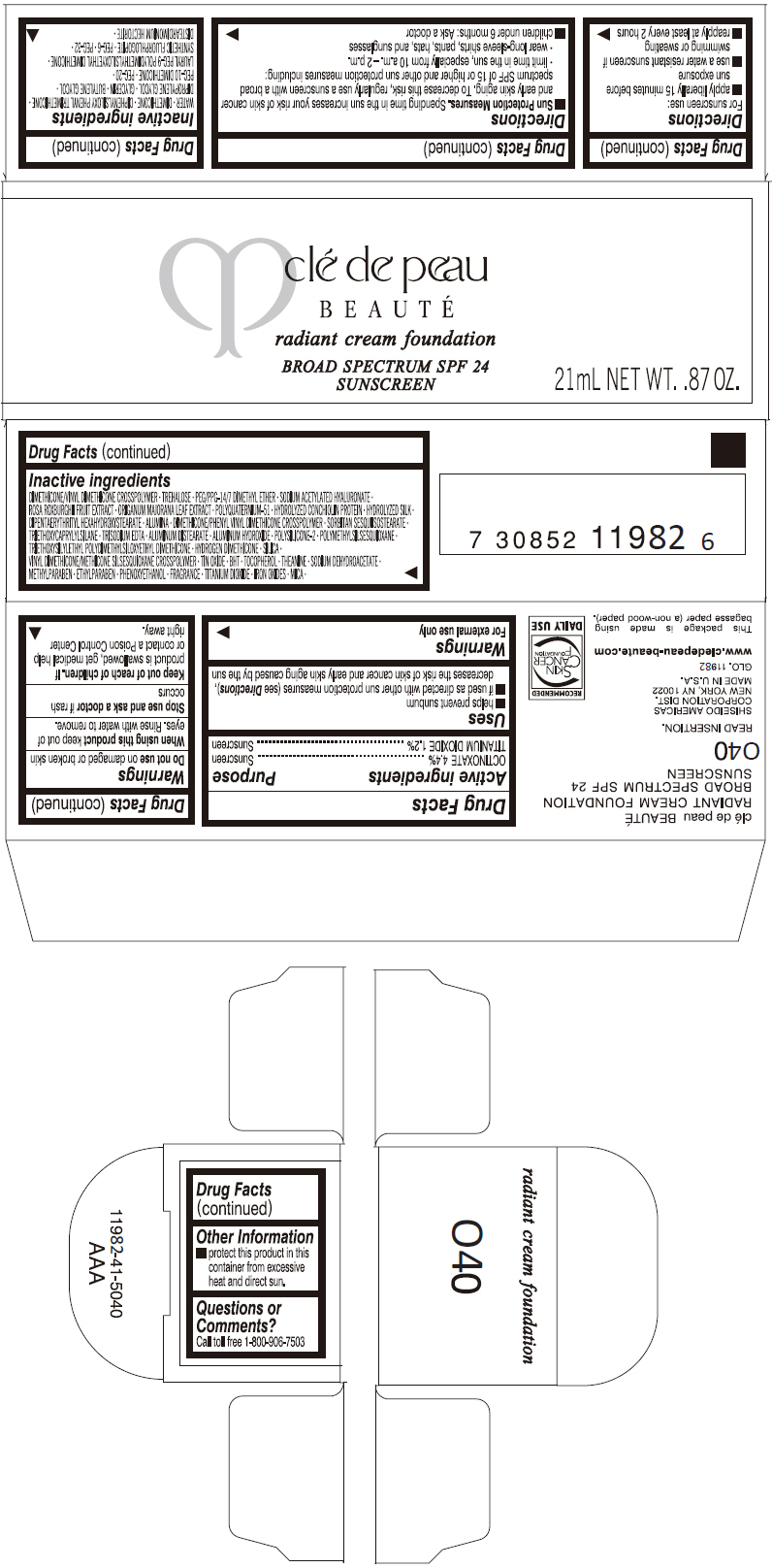
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
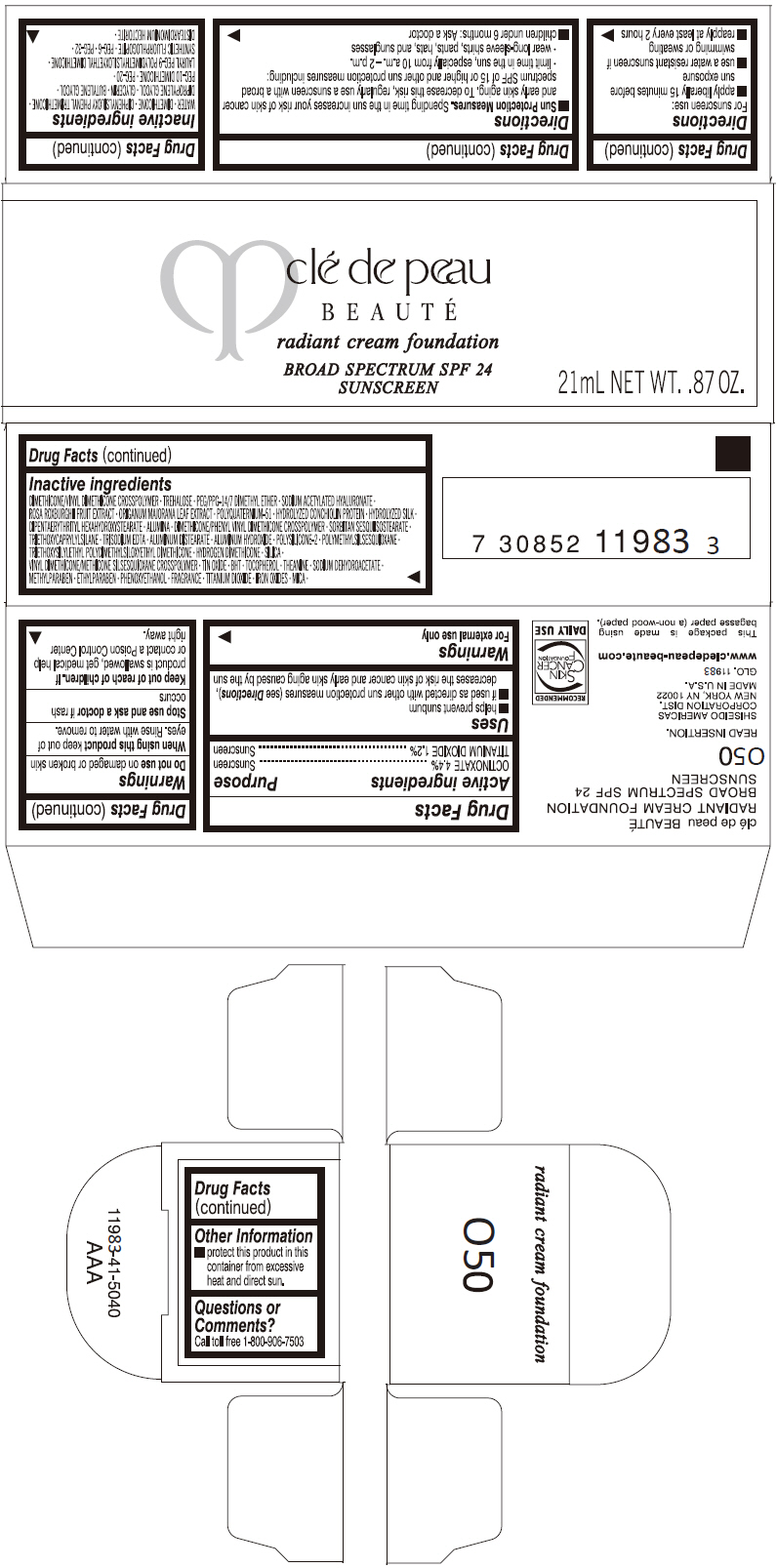
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.
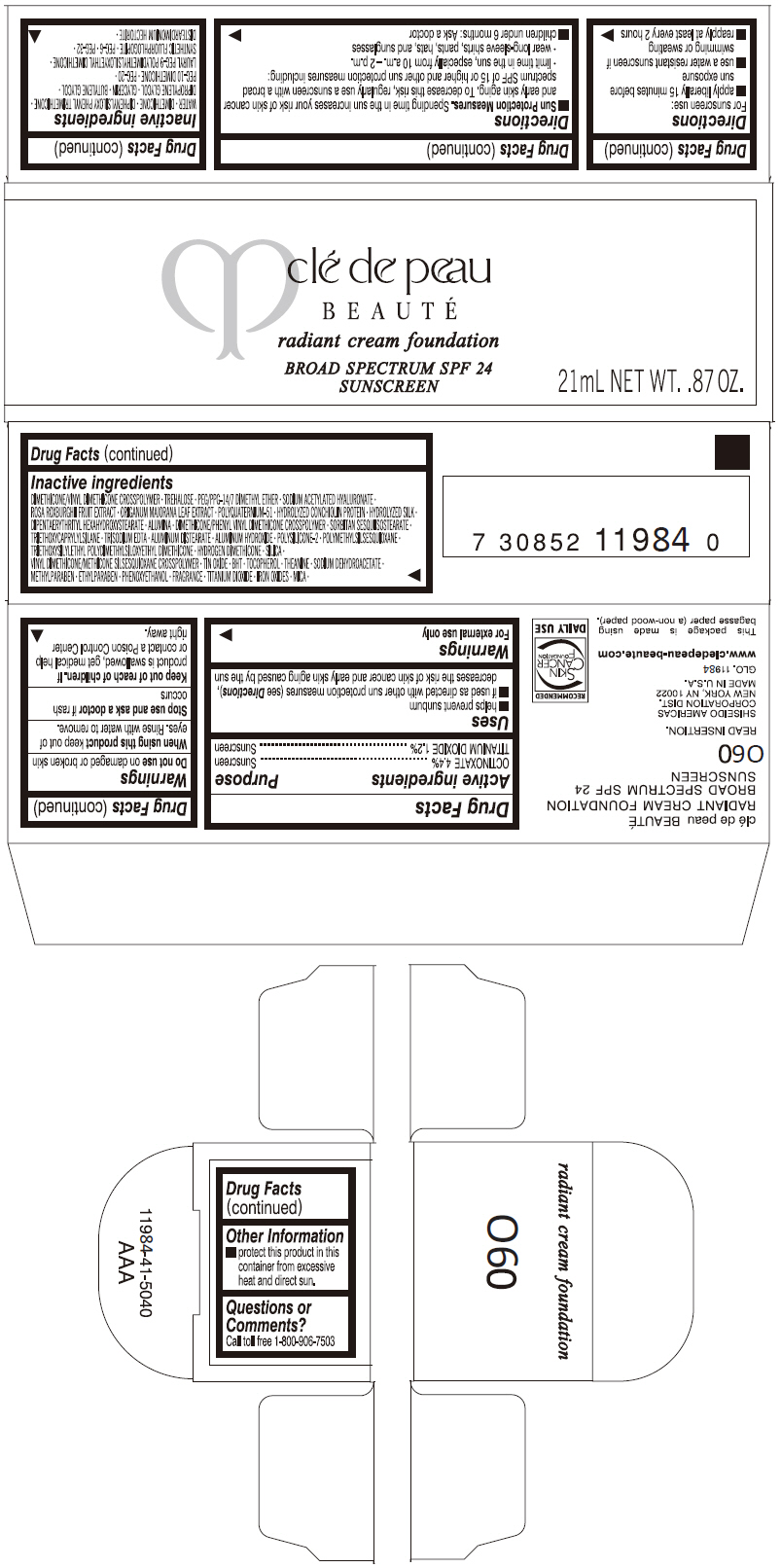
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant cream foundation
BROAD SPECTRUM SPF 24
SUNSCREEN
21mL NET WT. .87 OZ.