NDC Code(s) : 58414-2048-1
Packager : NeoStrata Company Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Exuviance Multi Protective Day Octinoxate and Titanium Dioxide CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
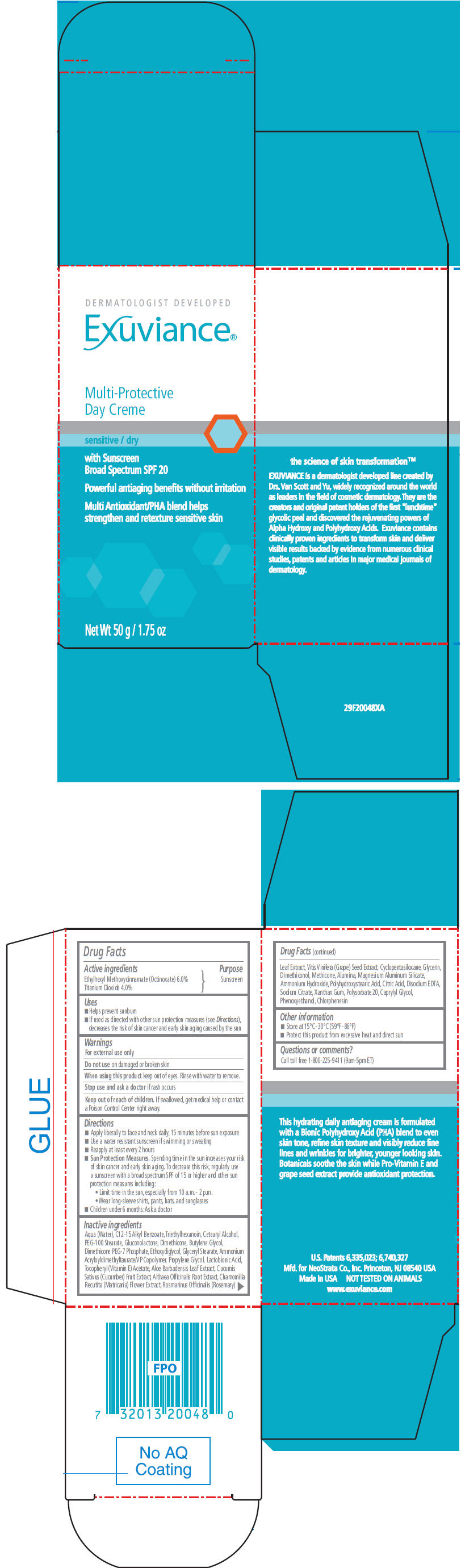
PRINCIPAL DISPLAY PANEL
DERMATOLOGIST DEVELOPED
Exuviance®
Multi-Protective
Day Creme
sensitive / dry
with Sunscreen
Broad Spectrum SPF 20
Powerful antiaging benefits without irritation
Multi Antioxidant/PHA blend helps
strengthen and retexture sensitive skin
Net Wt 50 g / 1.75 oz