NDC Code(s) : 58602-826-05, 58602-826-61, 58602-826-62
Packager : Aurohealth LLC
Category : Human OTC Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Acid Reducer Omeprazole TABLET, DELAYED RELEASE | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Aurohealth LLC(078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(58602-826), MANUFACTURE(58602-826) | |
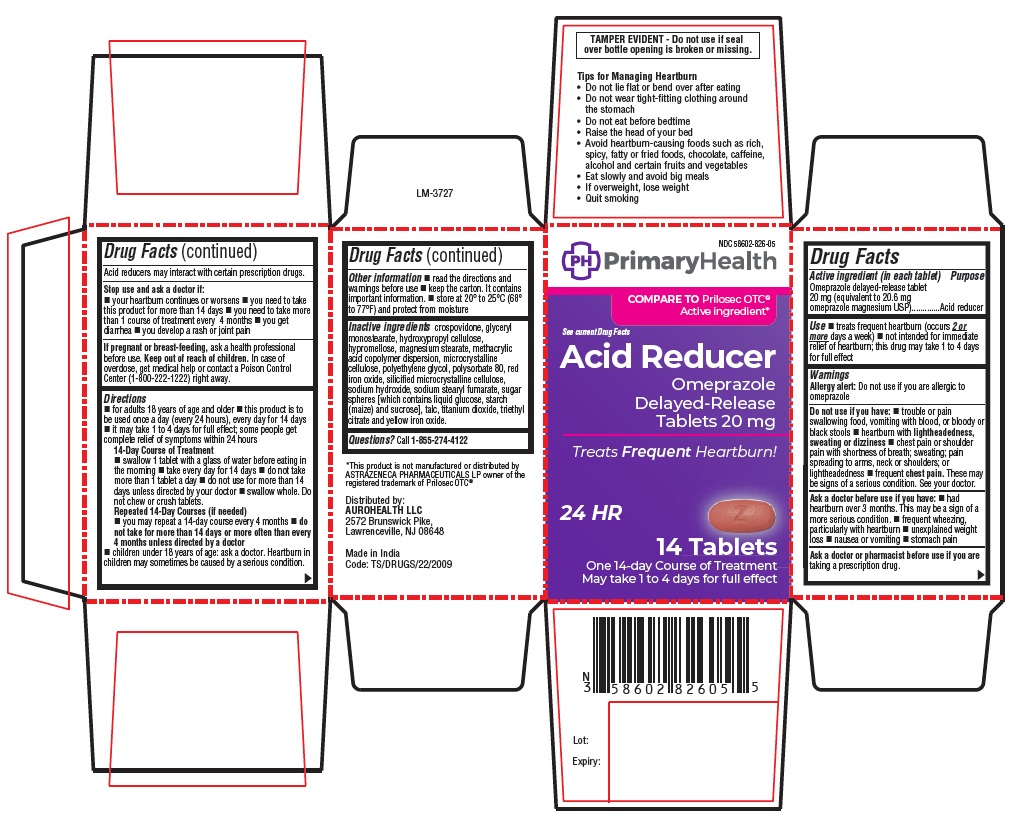
PRINCIPAL DISPLAY PANEL
NDC 58602-826-05 PrimaryHealth
See current Drug Facts
Acid Reducer
Omeprazole
Delayed-Release
Tablets 20 mg
Treats Frequent Heartburn!
24 HR
14 TABLETS
One 14-day course of treatment
May take 1 to 4 days for full effect

PRINCIPAL DISPLAY PANEL
NDC 58602-826-05
PrimaryHealth
COMPARE TO Prilosec OTC®
Active ingredient*
See current Drug Facts
ACID REDUCER
Omeprazole
Delayed-Release
Tablets 20 mg
Treats Frequent Heartburn!
24 HR
14 TABLETS
One 14-day Course of Treatment
May take 1 to 4 days for full effect