NDC Code(s) : 59011-751-04, 59011-758-04, 59011-752-04, 59011-750-04, 59011-757-04
Packager : Purdue Pharma LP
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Butransbuprenorphine PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Butransbuprenorphine PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Butransbuprenorphine PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Butransbuprenorphine PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Butransbuprenorphine PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Purdue Pharma LP(932323652) |
| REGISTRANT - Purdue Pharma LP(932323652) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Lohman Therapie System | 787660513 | MANUFACTURE(59011-757, 59011-750, 59011-752, 59011-751, 59011-758) | |
PRINCIPAL DISPLAY PANEL
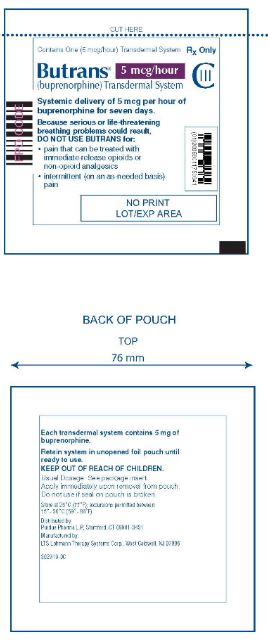
Butrans® 5 mcg Carton
NDC: 59011–750–04

Butrans® 5 mcg Pouch
NDC: 59011–750–04
PRINCIPAL DISPLAY PANEL
Butrans® 7.5 mcg Carton
NDC: 59011–757–04

Butrans® 7.5 mcg
Pouch
NDC: 59011–757–04

PRINCIPAL DISPLAY PANEL
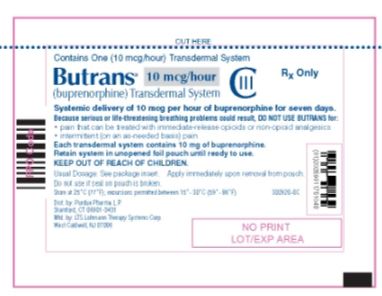
Butrans® 10 mcg Carton
NDC: 59011–751–04

Butrans® 10 mcg
Pouch
NDC: 59011–751–04
PRINCIPAL DISPLAY PANEL
Butrans® 15 mcg Carton
NDC: 59011–758–04

Butrans® 15 mcg
Pouch
NDC: 59011–758–04

PRINCIPAL DISPLAY PANEL
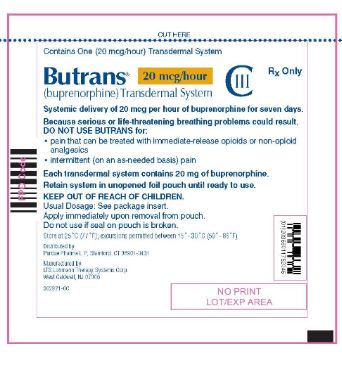
Butrans® 20 mcg Carton
NDC: 59011–752–04
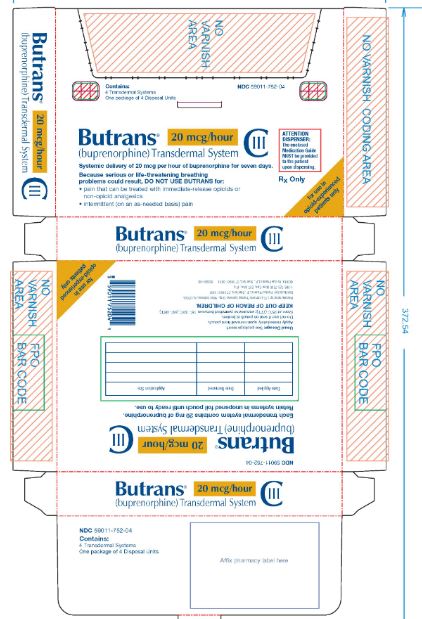
Butrans® 20 mcg
Pouch
NDC: 59011–752–04