NDC Code(s) : 59148-029-13, 59148-029-85, 59148-029-07, 59148-029-90, 59148-029-61, 59148-029-72, 59148-030-13, 59148-030-85, 59148-030-07, 59148-030-90, 59148-030-61, 59148-030-72, 59148-031-13, 59148-031-85, 59148-031-07, 59148-031-90, 59148-031-61, 59148-031-72, 59148-032-13, 59148-032-85, 59148-032-07, 59148-032-90, 59148-032-61, 59148-032-72, 59148-033-13, 59148-033-85, 59148-033-07, 59148-033-90, 59148-033-61, 59148-033-72, 59148-034-13, 59148-034-85, 59148-034-07, 59148-034-90, 59148-034-61, 59148-034-72
Packager : Otsuka America Pharmaceutical, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Abilify MyCiteARIPIPRAZOLE TABLET WITH SENSOR | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Abilify MyCiteARIPIPRAZOLE TABLET WITH SENSOR | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Abilify MyCiteARIPIPRAZOLE TABLET WITH SENSOR | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Abilify MyCiteARIPIPRAZOLE TABLET WITH SENSOR | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Abilify MyCiteARIPIPRAZOLE TABLET WITH SENSOR | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Abilify MyCiteARIPIPRAZOLE TABLET WITH SENSOR | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Otsuka America Pharmaceutical, Inc.(008314390) |
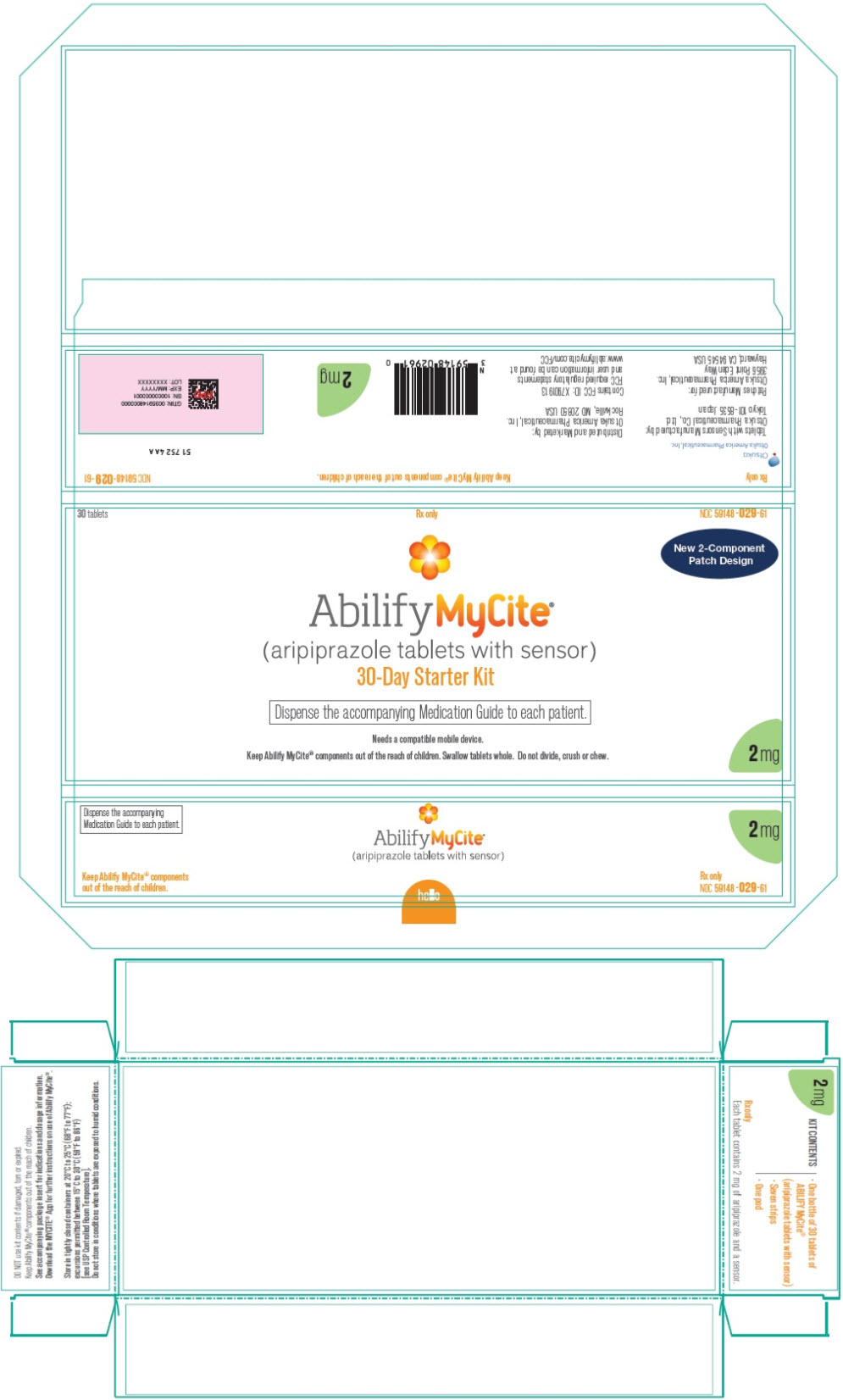
PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-029-61
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
30-Day Starter Kit
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
2 mg
PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-029-72
For
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
Maintenance Kit (tablets with sensor & strips)
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
2 mg

PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-030-61
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
30-Day Starter Kit
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
5 mg

PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-030-72
For
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
Maintenance Kit (tablets with sensor & strips)
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
5 mg

PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-031-61
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
30-Day Starter Kit
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
10 mg

PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-031-72
For
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
Maintenance Kit (tablets with sensor & strips)
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
10 mg

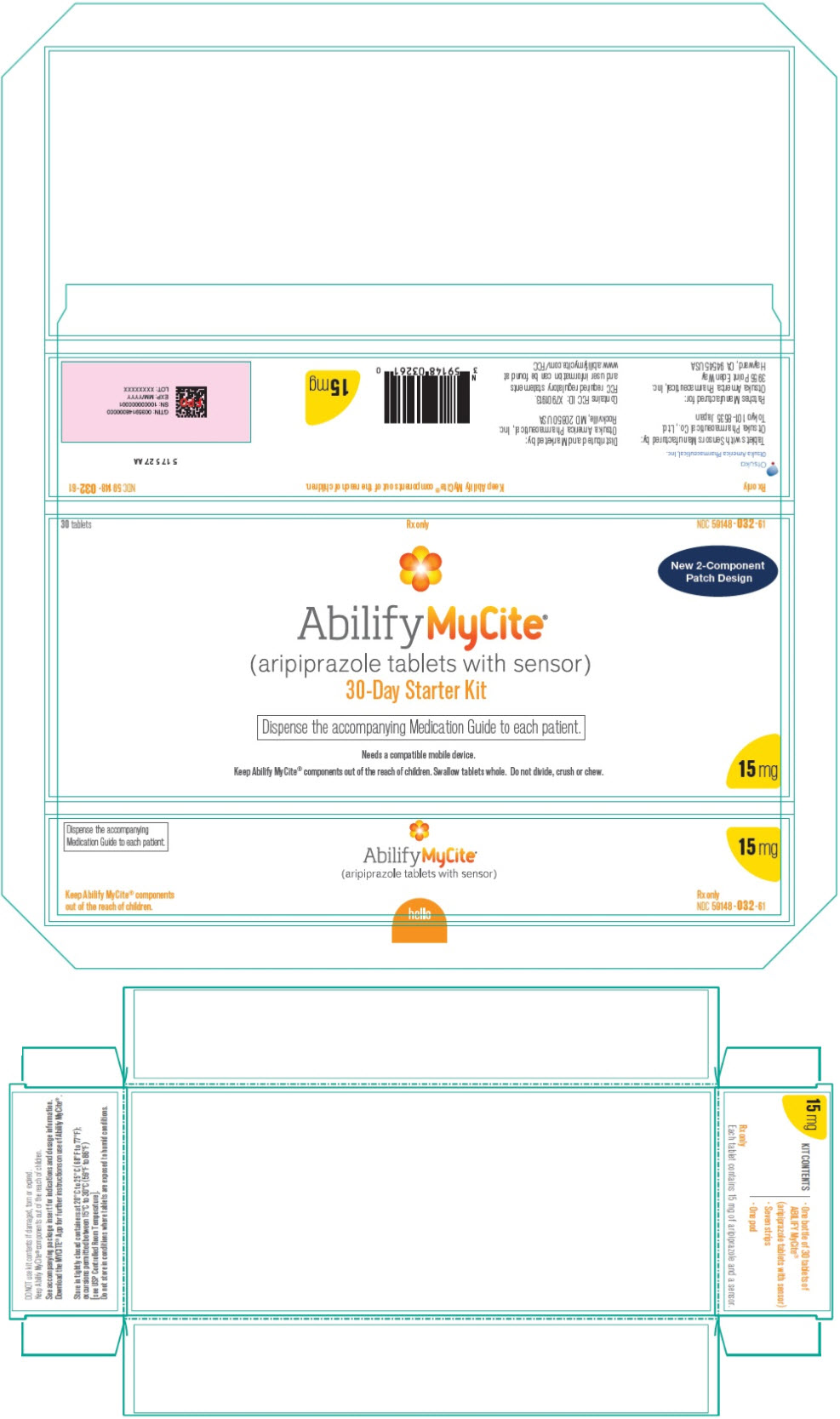
PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-032-61
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
30-Day Starter Kit
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
15 mg
PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-032-72
For
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
Maintenance Kit (tablets with sensor & strips)
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
15 mg

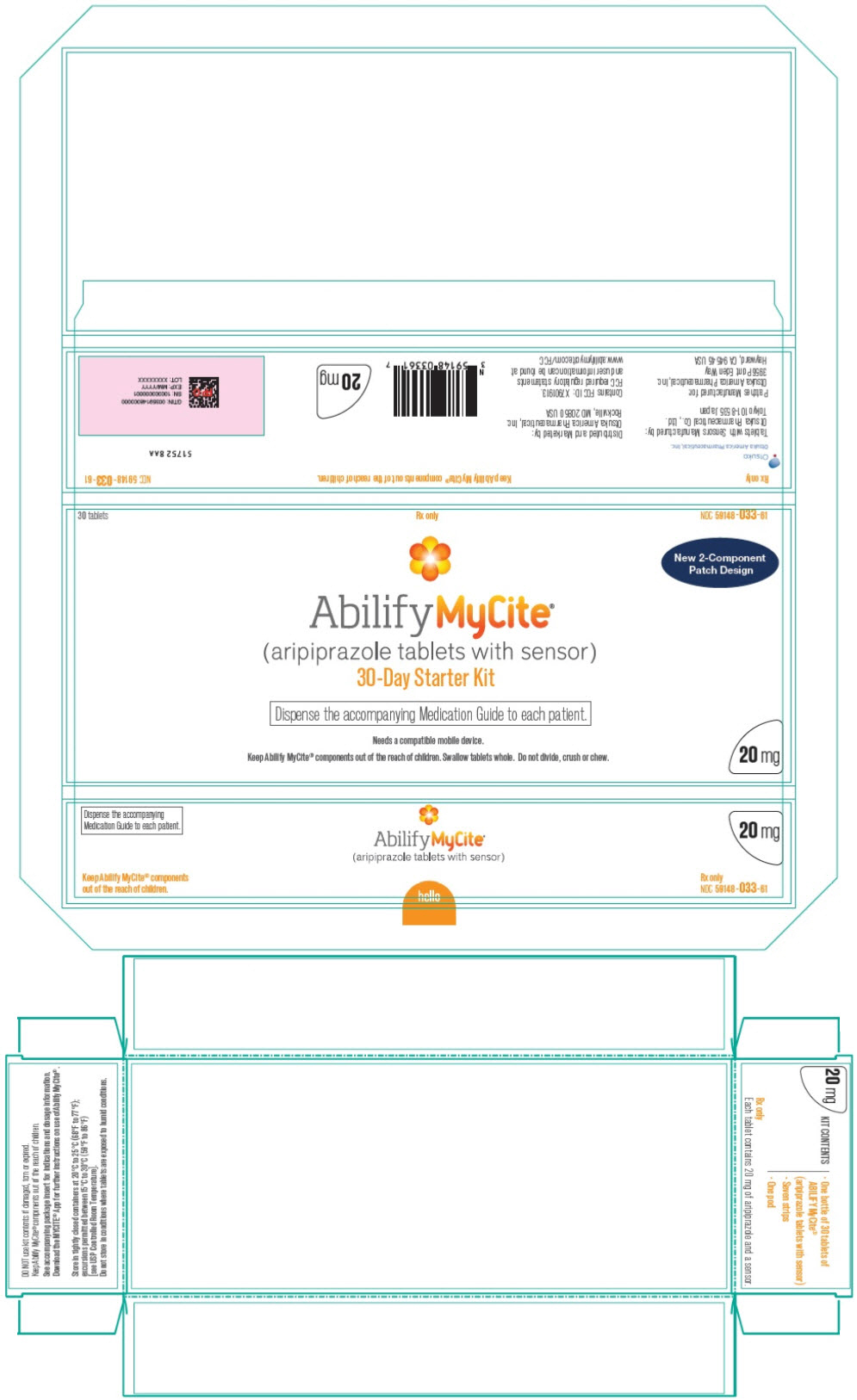
PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-033-61
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
30-Day Starter Kit
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
20 mg
PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-033-72
For
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
Maintenance Kit (tablets with sensor & strips)
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
20 mg

PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-034-61
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
30-Day Starter Kit
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
30 mg

PRINCIPAL DISPLAY PANEL
30 tablets
Rx only
NDC 59148-034-72
For
New 2-Component
Patch Design
Abilify MyCite®
(aripiprazole tablets with sensor)
Maintenance Kit (tablets with sensor & strips)
Dispense the accompanying Medication Guide to each patient.
Needs a compatible mobile device.
Keep Abilify MyCite® components out of the reach of children. Swallow tablets whole. Do not divide, crush or chew.
30 mg










