NDC Code(s) : 59148-070-90, 59148-070-91
Packager : Otsuka America Pharmaceutical, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BUSULFEXbusulfan INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Otsuka America Pharmaceutical, Inc.(008314390) |
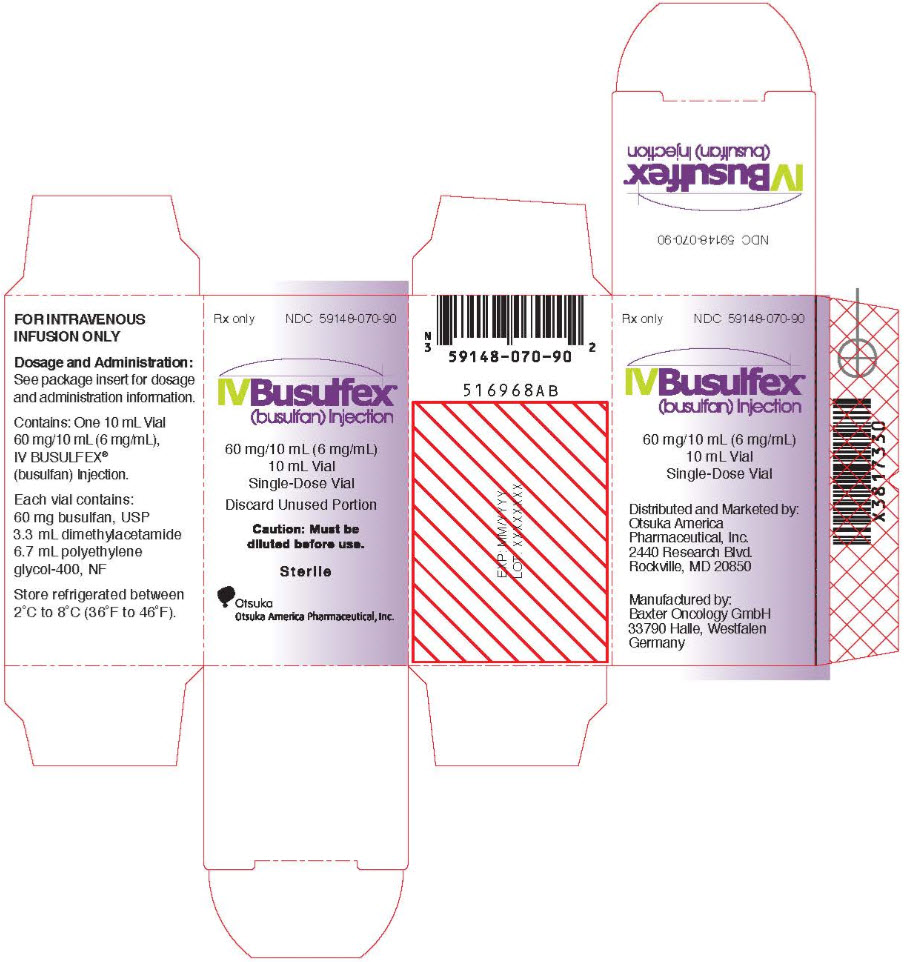
PRINCIPAL DISPLAY PANEL
Rx only
NDC 59148-070-90
Sterile
IVBusulfex®
(busulfan) Injection
60 mg/10 mL (6 mg/mL)
10 mL Vial
Single-Dose Vial
Discard Unused Portion
Caution: Must be
diluted before use.

PRINCIPAL DISPLAY PANEL
Rx only
NDC 59148-070-90
IVBusulfex®
(busulfan) Injection
60 mg/10 mL (6 mg/mL)
10 mL Vial
Single-Dose Vial
Discard Unused Portion
Caution: Must be
diluted before use.
Sterile
Otsuka
Otsuka America Pharmaceutical, Inc.