NDC Code(s) : 59310-610-31, 59310-610-33
Packager : Teva Respiratory, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CINQAIRReslizumab INJECTION, SOLUTION, CONCENTRATE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Teva Respiratory, LLC(176697907) |
PRINCIPAL DISPLAY PANEL
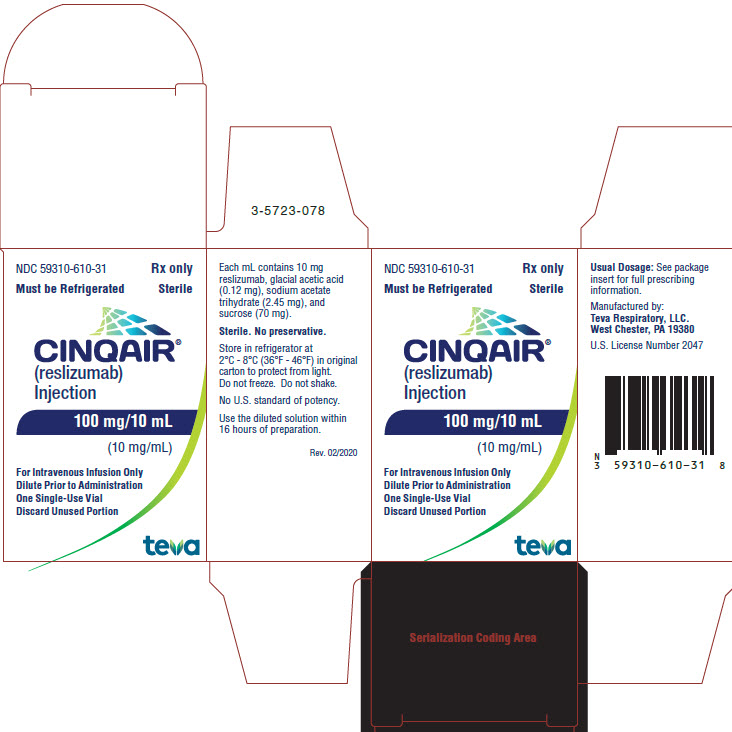
NDC 59310-610-31
Rx only
Must be Refrigerated Sterile
CINQAIR® (reslizumab) Injection 100 mg/10 mL (10 mg/mL)
For Intravenous Infusion Only
Dilute Prior to Administration
One Single-Use Vial
Discard Unused Portion
TEVA
PRINCIPAL DISPLAY PANEL
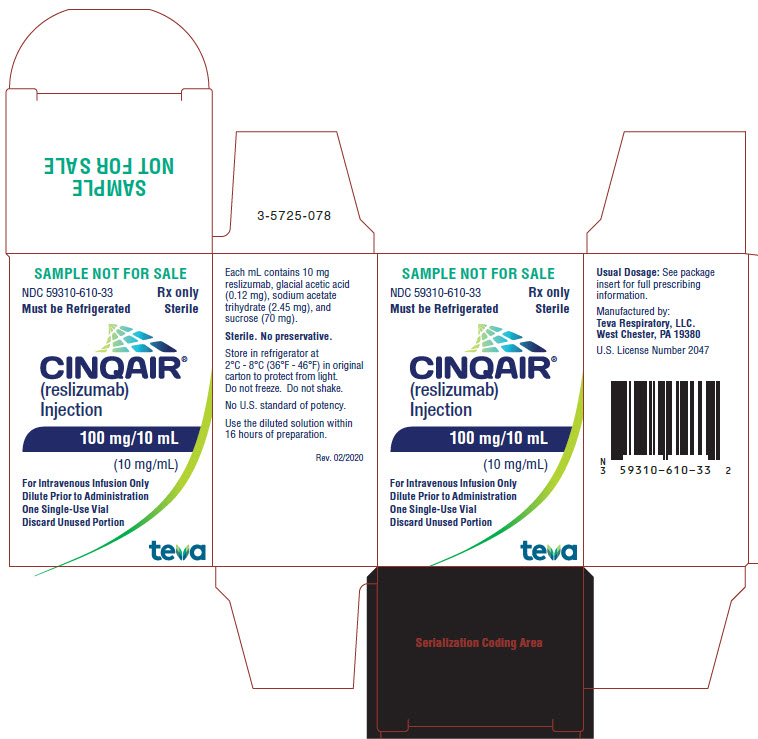
NDC 59310-610-33
Rx only
Must be Refrigerated
Sterile
CINQAIR® (reslizumab) Injection 100 mg/10 mL (10 mg/mL)
For Intravenous Infusion Only
Dilute Prior to Administration
One Single-Use Vial
Discard Unused Portion
TEVA









