NDC Code(s) : 59316-104-50
Packager : Performance Health LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Biofreeze MENTHOL SPRAY | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
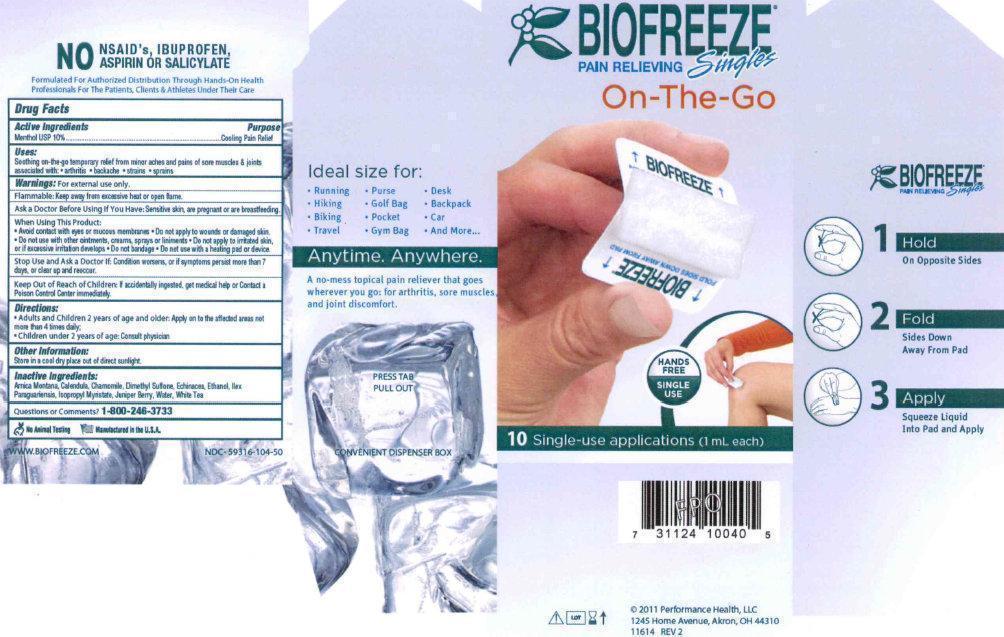
PRINCIPAL DISPLAY PANEL
BIOFREEZE PAIN RELIEVING Singles On-The-Go HANDS FREE SINGLE USE 10 Single-use applications (1 mL each) 2011 Performance Health, LLC 1245 Home Avenue, Akron, OH 44310
PRINCIPAL DISPLAY PANEL