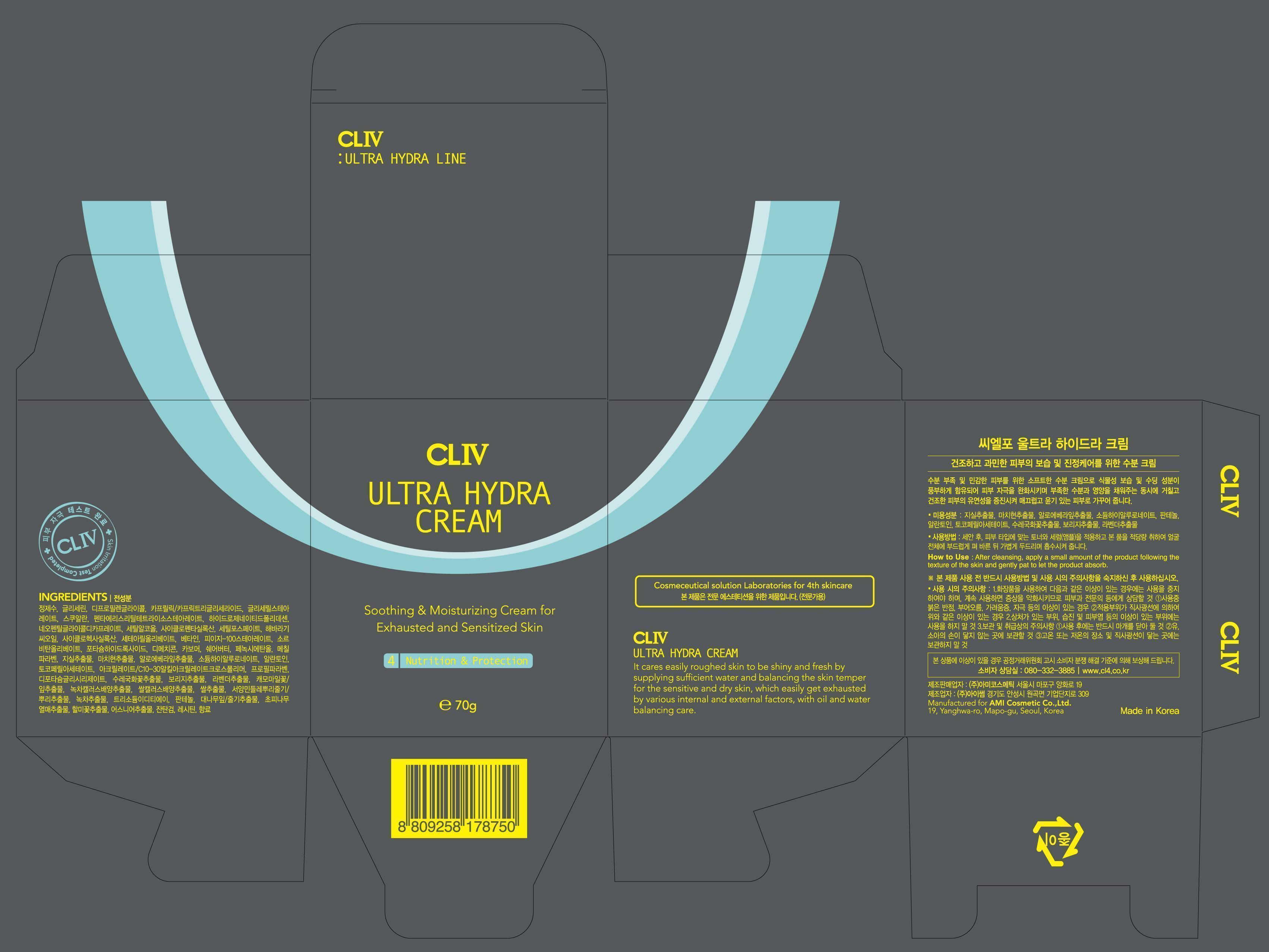
NDC Code(s) : 59535-2701-1
Packager : AMI Cosmetic Co.,Ltd.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ULTRA HYDRA GLYCERIN CREAM | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
PRINCIPAL DISPLAY PANEL