NDC Code(s) : 59627-333-04, 59627-222-05, 59627-003-01, 59627-002-07, 59627-002-06
Packager : Biogen Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AVONEXinterferon beta-1a KIT | ||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| AVONEXinterferon beta-1a KIT | ||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Avonex PenInterferon Beta-1a INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| AvonexInterferon Beta-1a INJECTION, SOLUTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Biogen Inc.(009492211) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Packaging Coordinators, LLC | 078525133 | LABEL(59627-003, 59627-002, 59627-333, 59627-222), PACK(59627-003, 59627-002, 59627-333, 59627-222) | |
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 30 mcg Carton Label
NDC 59627-003-01
Avonex Pen ®
(interferon beta-1a)
Injection
30 mcg/0.5 mL Single-Use Prefilled Autoinjector
For Intramuscular Injection
Once a Week
Avonex Pen® Administration Dose Pack
ATTENTION PHARMACIST: Each patient is required to receive the enclosed Medication Guide.
Store refrigerated at 2-8°C (36-46°F).
Do not freeze or expose to high temperatures. Protect from light.
The recommended dosage of Avonex® is 30 mcg injected intramuscularly
once weekly. See package insert for full prescribing information.
This Product Contains Dry Natural Rubber.
Rx only

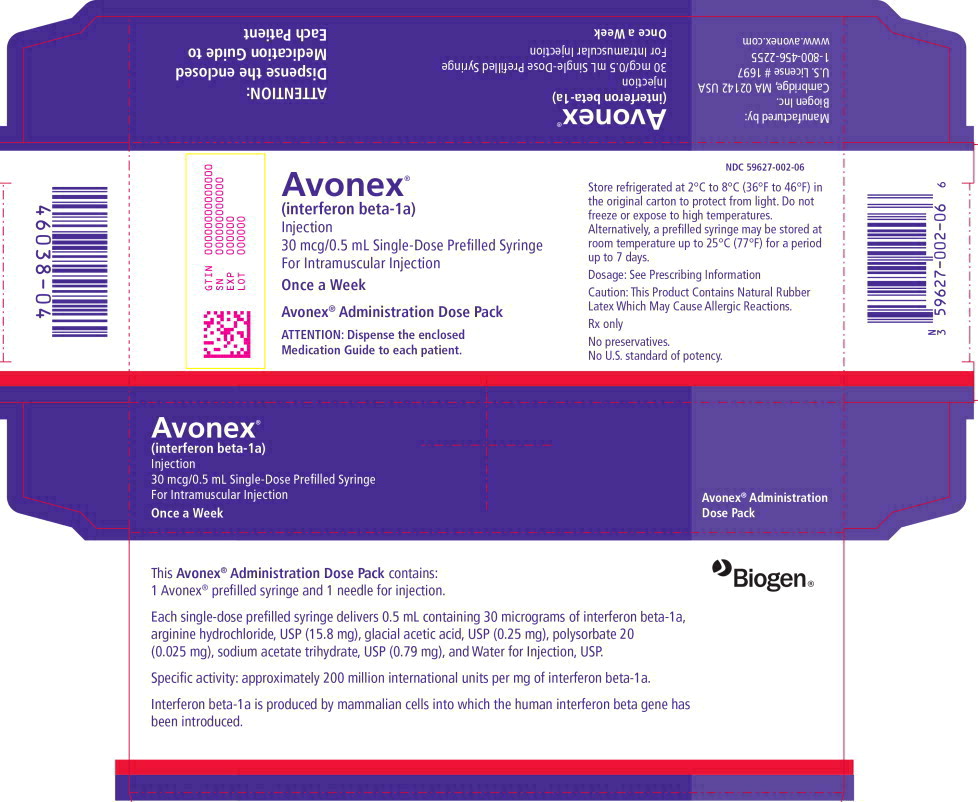
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 30 mcg Carton Label
NDC 59627-002-06
Avonex
®
(interferon beta-1a)
Injection
30 mcg/0.5 mL Single-Use Prefilled Syringe
For Intramuscular Injection
Once a Week
Avonex ® Administration Dose Pack
ATTENTION PHARMACIST: Each patient is required
to receive the enclosed Medication Guide.
Store refrigerated at 2-8°C (36-46°F)
Do not freeze or expose to high temperatures.
Protect from light.
See package insert for dosage and administration.
This Product Contains Dry Natural Rubber.
Rx only
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Carton Label
NDC 59627-333-04
Avonex Pen
®
(interferon beta-1a)
Injection
30 mcg/0.5 mL Single-Use Prefilled Autoinjector
For Intramuscular Injection
Once a Week
Contents:
4 Avonex Pen ® Administration Dose Packs
Store refrigerated at 2-8°C (36-46°F)
Do not freeze or expose to high temperatures. Protect from light.
The recommended dosage of Avonex
® is 30 mcg injected intramuscularly
once weekly. See package insert for full prescribing information.
ATTENTION PHARMACIST: Each patient is required to receive the enclosed Medication Guide.

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Carton Label
NDC 59627-222-05
Avonex
®
(interferon beta-1a)
Injection
30 mcg/0.5 mL Single-Use Prefilled Syringe
For Intramuscular Injection
Once a Week
Contents:
4 Avonex ® Administration Dose Packs
ATTENTION PHARMACIST: Each patient is required to receive the enclosed Medication Guide.
This Product Contains Dry Natural Rubber.
Rx only
Store refrigerated at 2-8°C (36-46°F).
Do not freeze or expose to high temperatures.
Protect from light.
See package insert for dosage and administration.

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Tray Lid Label
Avonex ® (interferon beta-1a)
30 mcg/0.5 mL Single-Use Prefilled Syringe
For Intramuscular Injection
Store refrigerated at 2-8°C (36-46°F). Do not freeze or expose to high temperatures.
Protect from light. Contents: 1 single-use prefilled syringe of Avonex® and 1, 23G, 1 1/4" needle. See package insert for dosage and administration. This Product Contains Dry Natural Rubber. ATTENTION PHARMACIST: Each patient is required to receive the enclosed Medication Guide. U.S. License# 1697
Biogen Inc., Cambridge, MA 02142 1-800-456-2255 Rx only 46043 -02
NDC 59627-002-07










