NDC Code(s) : 59741-116-06, 59741-116-10
Packager : Bio-Pharm, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Tussin DMGuaifenesin and Dextromethorphan Hydrobromide SOLUTION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
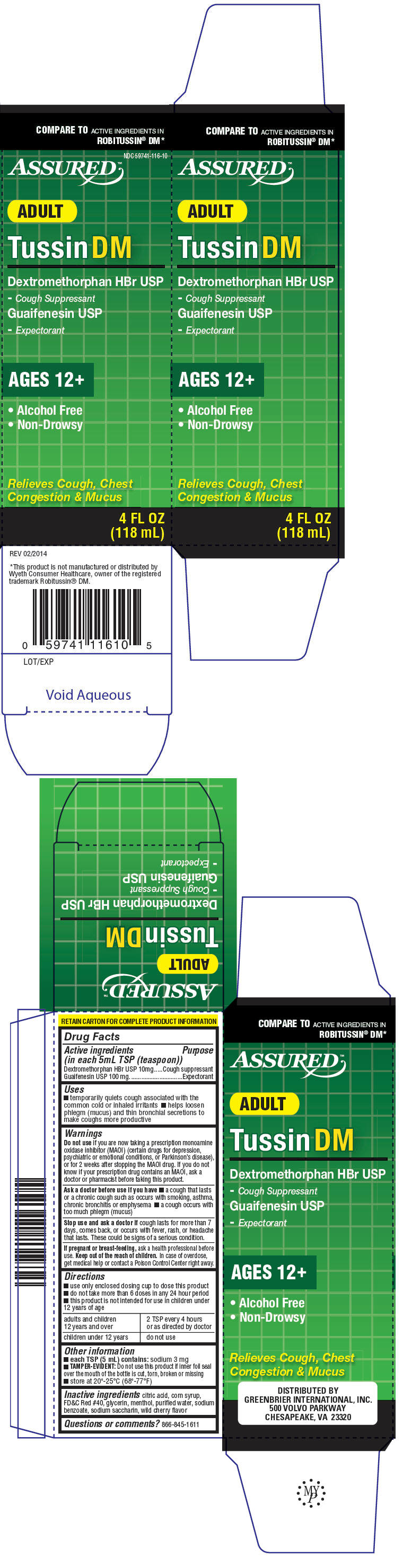
PRINCIPAL DISPLAY PANEL
COMPARE TO ACTIVE INGREDIENTS IN
ROBITUSSIN
® DM*
NDC 59741-116-10
ASSURED™
ADULT
Tussin DM
Dextromethorphan HBr USP
- Cough Suppressant
Guaifenesin USP
- Expectorant
AGES 12+
- Alcohol Free
- Non-Drowsy
Relieves Cough, Chest
Congestion & Mucus
4 FL OZ
(118 mL)