NDC Code(s) : 59741-246-04
Packager : Bio-Pharm, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Childrens Pain ReliefAcetaminophen LIQUID | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
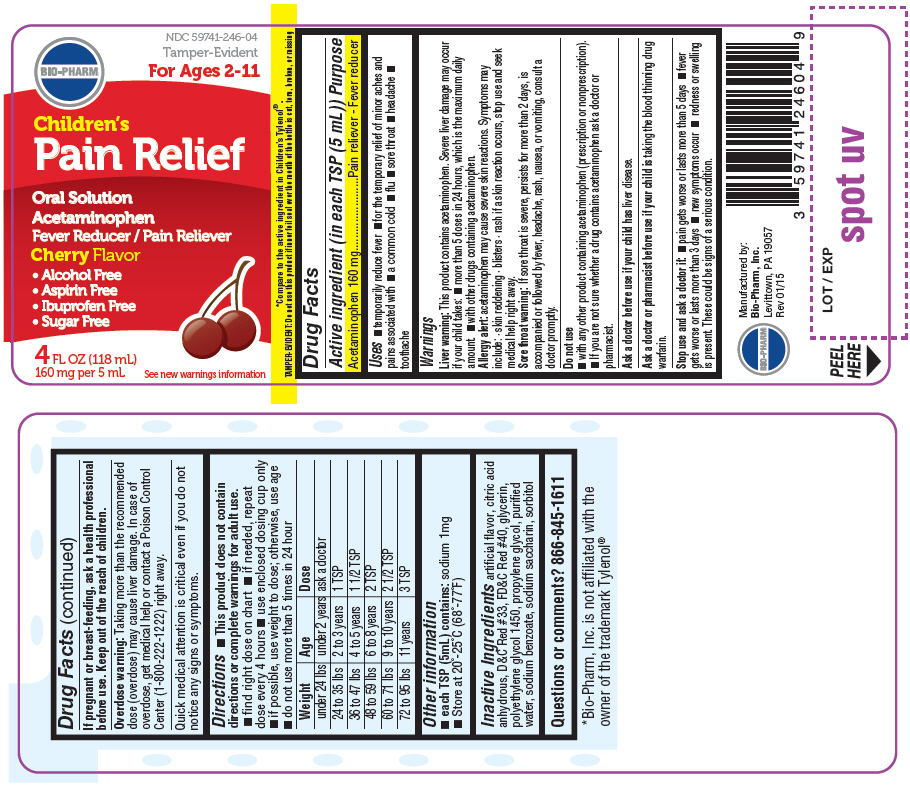
PRINCIPAL DISPLAY PANEL
NDC 59741-246-04
Tamper-Evident
For Ages 2-11
BIO-PHARM
Children's
Pain Relief
Oral Solution
Acetaminophen
Fever Reducer / Pain Reliever
Cherry Flavor
- Alcohol Free
- Aspirin Free
- Ibuprofen Free
- Sugar Free
4 FL OZ (118 mL)
160 mg per 5 mL
See new warnings information