NDC Code(s) : 59779-385-03
Packager : CVS Pharmacy
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Benzethonium chloride Plus Dyclonine hydrochlorideLiquid Bandage LIQUID | ||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| LABELER - CVS Pharmacy(062312574) |
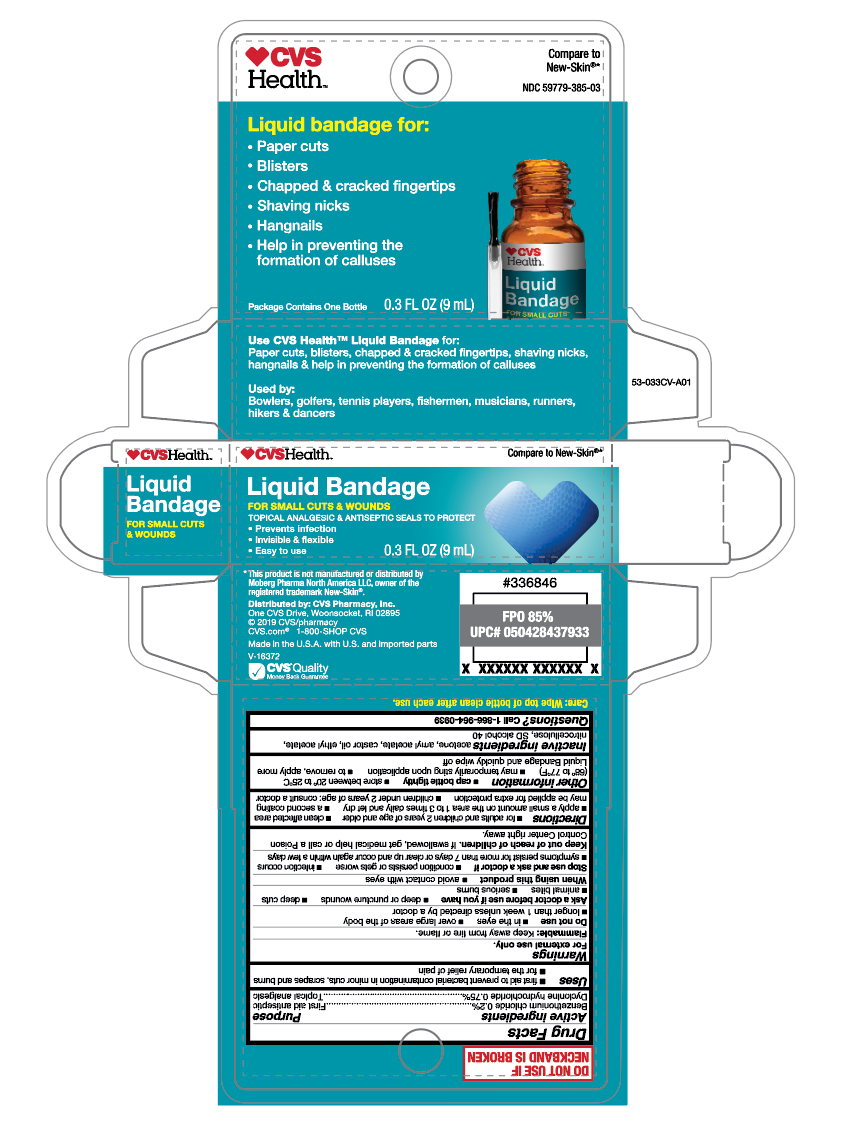
PRINCIPAL DISPLAY PANEL
CVS Health
Liquid Bandage for:
- Paper cuts
- Blisters
- Chapped & cracked fingertips
- Shaving nicks
- Hangnails
- Help in preventing the formation of calluses
Package Contains One Bottle
0.3 FL OZ (9mL)