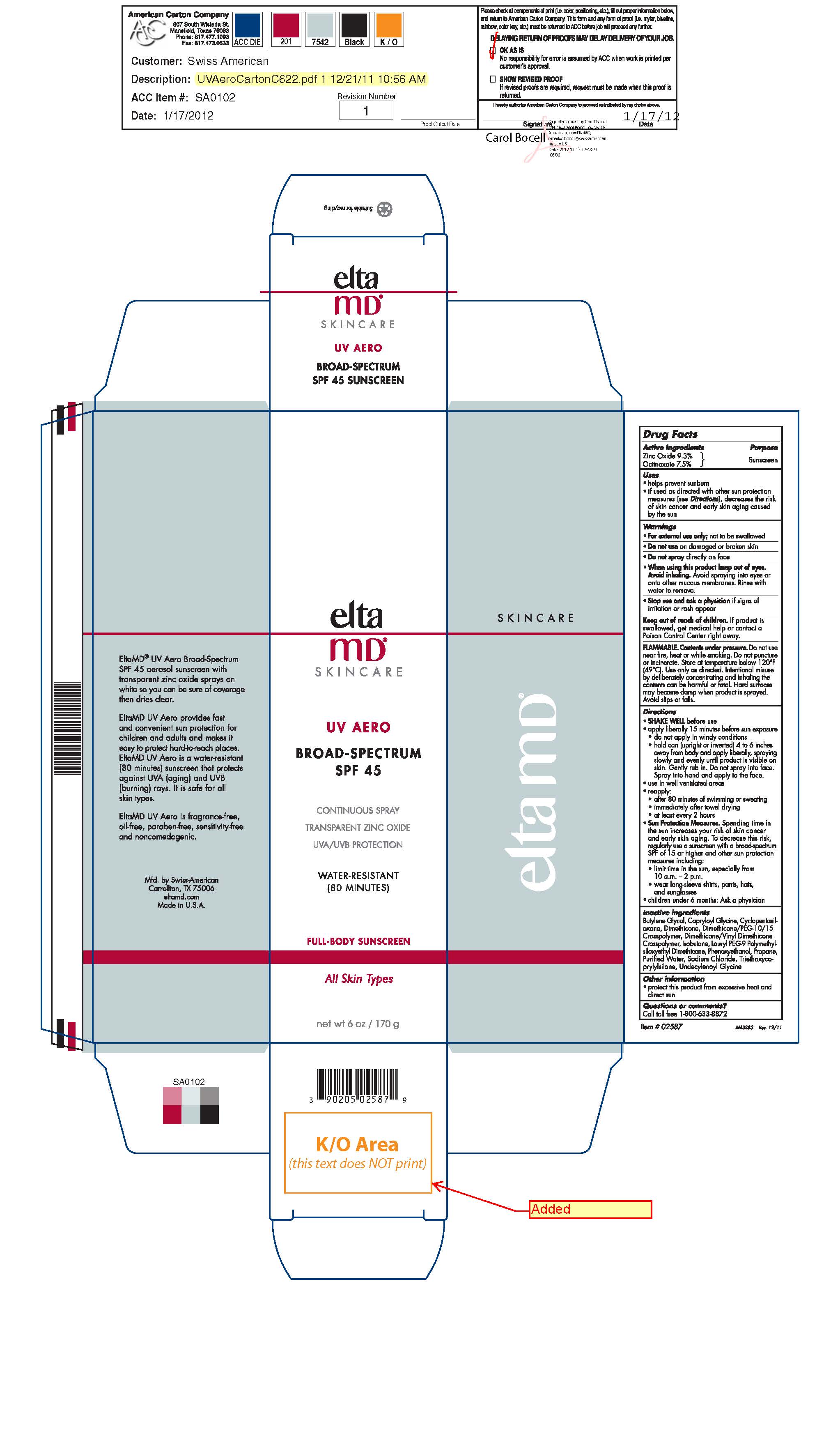
NDC Code(s) : 60232-2587-6
Packager : Swiss American Products, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Elta MD UV Aero zinc oxide and octinoxate aerosol AEROSOL, SPRAY | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL