NDC Code(s) : 60429-355-37, 60429-356-21
Packager : Golden State Medical Supply, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CHOLESTYRAMINEcholestyramine POWDER, FOR SUSPENSION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CHOLESTYRAMINE LIGHTcholestyramine light POWDER, FOR SUSPENSION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
PRINCIPAL DISPLAY PANEL
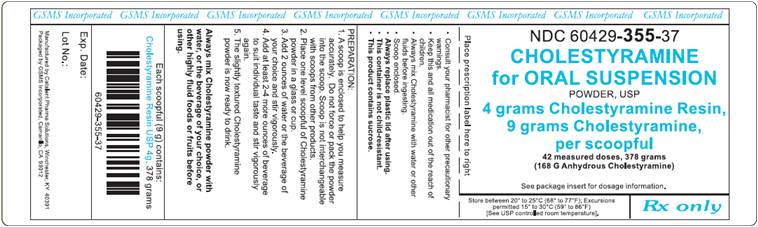
PRINCIPAL DISPLAY PANEL










