NDC Code(s) : 60429-371-01, 60429-371-10, 60429-372-10, 60429-372-01, 60429-373-01, 60429-373-10, 60429-374-01, 60429-374-10, 60429-375-60, 60429-376-01
Packager : Golden State Medical Supply, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| quetiapine fumaratequetiapine fumarate TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| quetiapine fumaratequetiapine fumarate TABLET, FILM COATED | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| quetiapine fumaratequetiapine fumarate TABLET, FILM COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| quetiapine fumaratequetiapine fumarate TABLET, FILM COATED | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| quetiapine fumaratequetiapine fumarate TABLET, FILM COATED | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| quetiapine fumaratequetiapine fumarate TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
APOTEX CORP. NDC 60429-371-01
Quetiapine Fumarate Tablets
25 mg
Rx only
100 Tablets

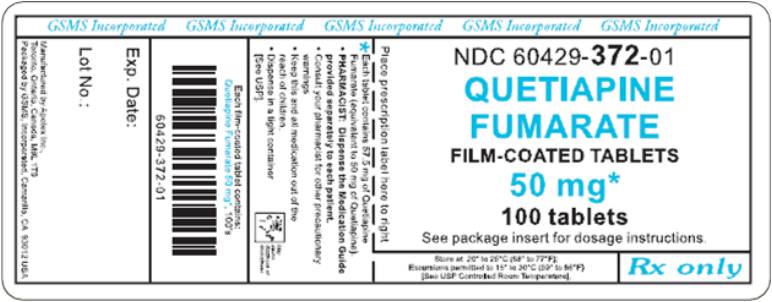
PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
APOTEX CORP. NDC 60429-372-01
Quetiapine Fumarate Tablets
50 mg
Rx only
100 Tablets
PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
APOTEX CORP. NDC 60429-373-01
Quetiapine Fumarate Tablets
100 mg
Rx only
100 Tablets

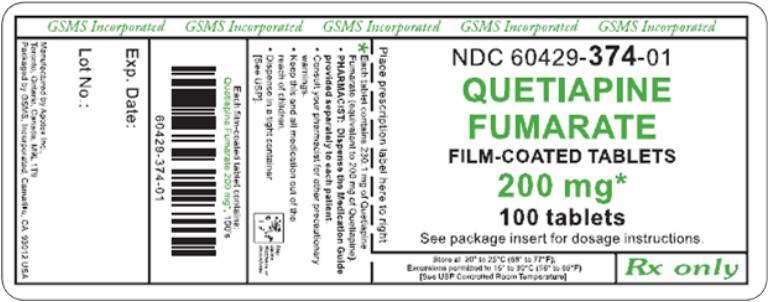
PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
APOTEX CORP. NDC 60429-374-01
Quetiapine Fumarate Tablets
200 mg
Rx only
100 Tablets
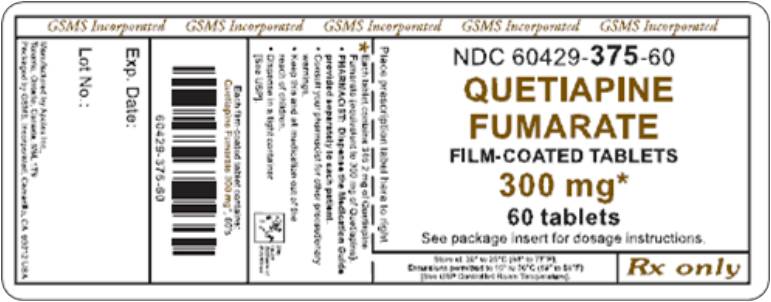
PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
APOTEX CORP. NDC 60429-375-60
Quetiapine Fumarate Tablets
300 mg
Rx only
60 Tablets
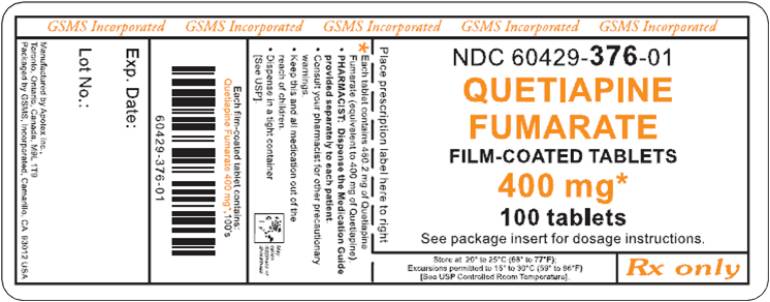
PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
APOTEX CORP. NDC 60429-376-01
Quetiapine Fumarate Tablets
400 mg
Rx only
100 Tablets