NDC Code(s) : 60505-0597-1, 60505-0597-2, 60505-0597-3, 60505-0598-1, 60505-0598-2, 60505-0598-3
Packager : Apotex Corp.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Timolol MaleateTimolol Maleate SOLUTION/ DROPS | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Timolol MaleateTimolol Maleate SOLUTION/ DROPS | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
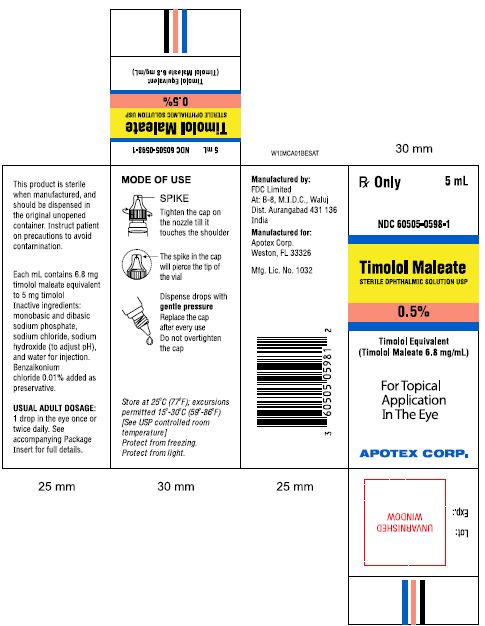
PRINCIPAL DISPLAY PANEL
Rx Only 5mL
NDC 60505-0597-1
Timolol Maleate
STERILE OPTHALMIC SOLUTION USP
0.25%
Timolol Equivalent (Timolol Maleate 3.4 mg/mL)
For Topical Application In The Eye
APOTEX CORP.
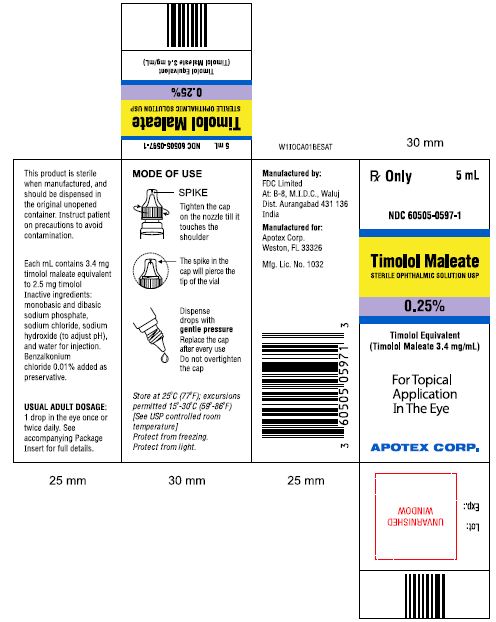
PRINCIPAL DISPLAY PANEL
Rx Only 5mL
NDC 60505-0598-1
Timolol Maleate
STERILE OPTHALMIC SOLUTION USP
0.5%
Timolol Equivalent (Timolol Maleate 6.8 mg/mL)
For Topical Application In The Eye
APOTEX CORP.