NDC Code(s) : 60505-6245-4, 60505-6246-4
Packager : Apotex Corp.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CefepimeCefepime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CefepimeCefepime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Apotex Corp.(845263701) |
| REGISTRANT - Qilu Pharmaceutical Co., Ltd.(653878256) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Qilu Pharmaceutical Co., Ltd. (High-Tech Zone Site) | 421279342 | manufacture(60505-6245, 60505-6246), analysis(60505-6245, 60505-6246) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Qilu Antibiotics Pharmaceutical Co., Ltd. | 527271779 | manufacture(60505-6245, 60505-6246), analysis(60505-6245, 60505-6246) | |
PRINCIPAL DISPLAY PANEL
NDC 60505-6245-0 Rx Only
Cefepime
for Injection, USP
1 gram/vial
Single-Dose Vial
Reconstitute before Intramuscular Use.
Dilute before Intravenous Infusion.
P R E M I E R P r o R x ®


PRINCIPAL DISPLAY PANEL
NDC 60505-6246-0 Rx Only
Cefepime
for Injection, USP
2 grams per vial
Single-Dose Vial
Dilute before Intravenous Infusion.
P R E M I E R P r o R x ®


PRINCIPAL DISPLAY PANEL
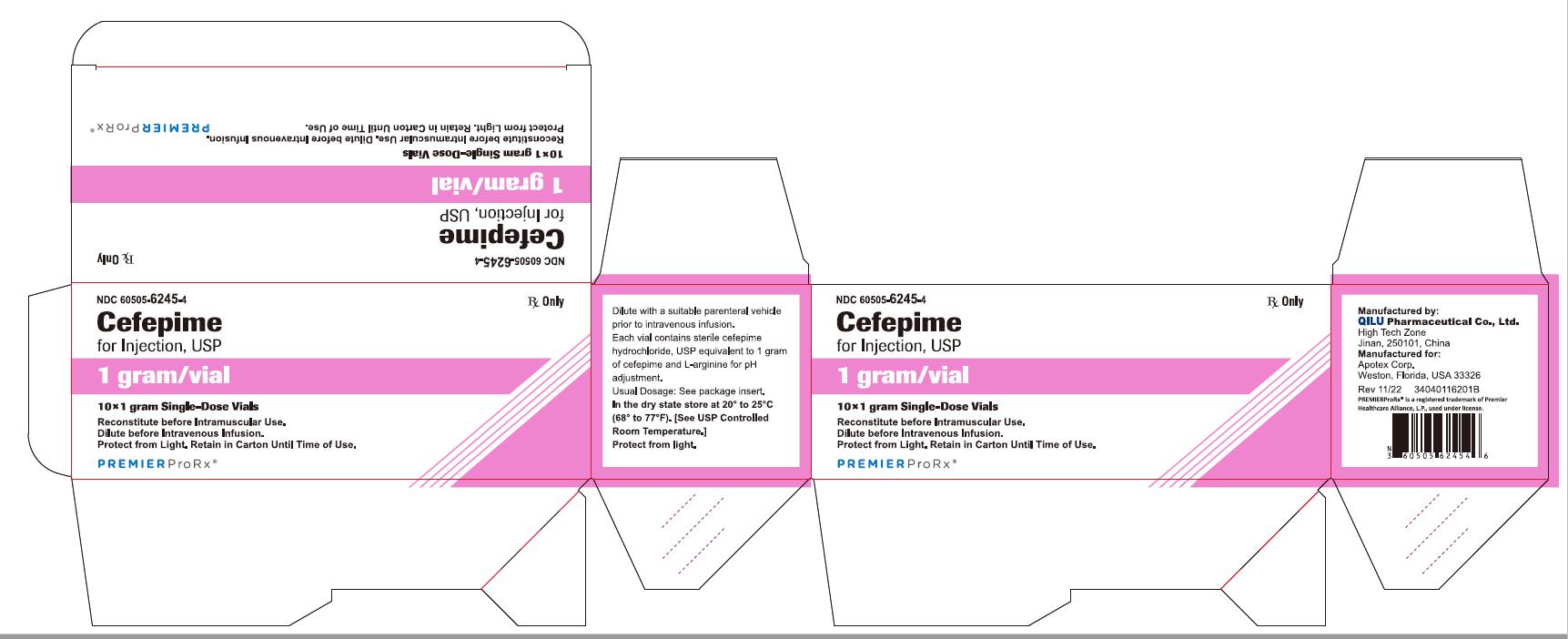
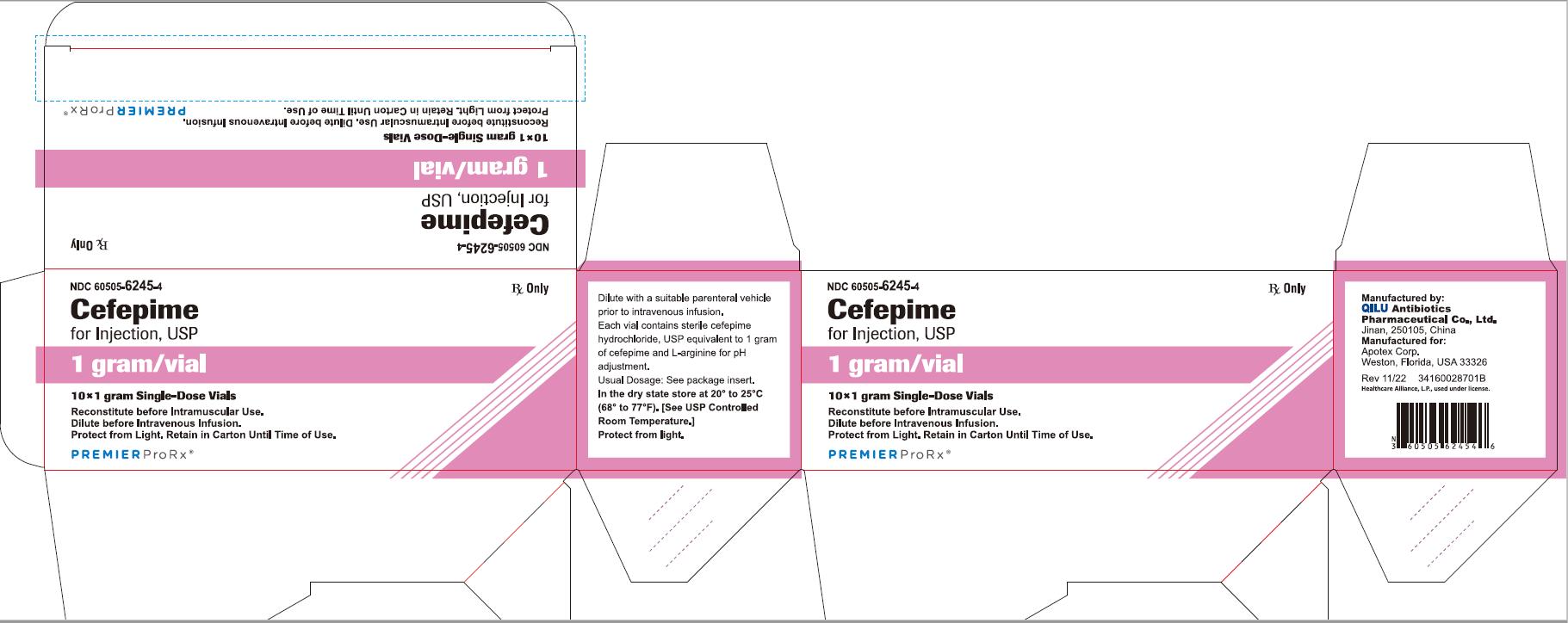
NDC 60505-6245-4 Rx Only
Cefepime
for Injection, USP
1 gram per vial
10*1 gram Single-Dose Vials
Reconstitute before Intramuscular Use.
Dilute before Intravenous Infusion.
Protect from Light. Retain in Carton Until Time of Use.
P R E M I E R P r o R x ®
PRINCIPAL DISPLAY PANEL
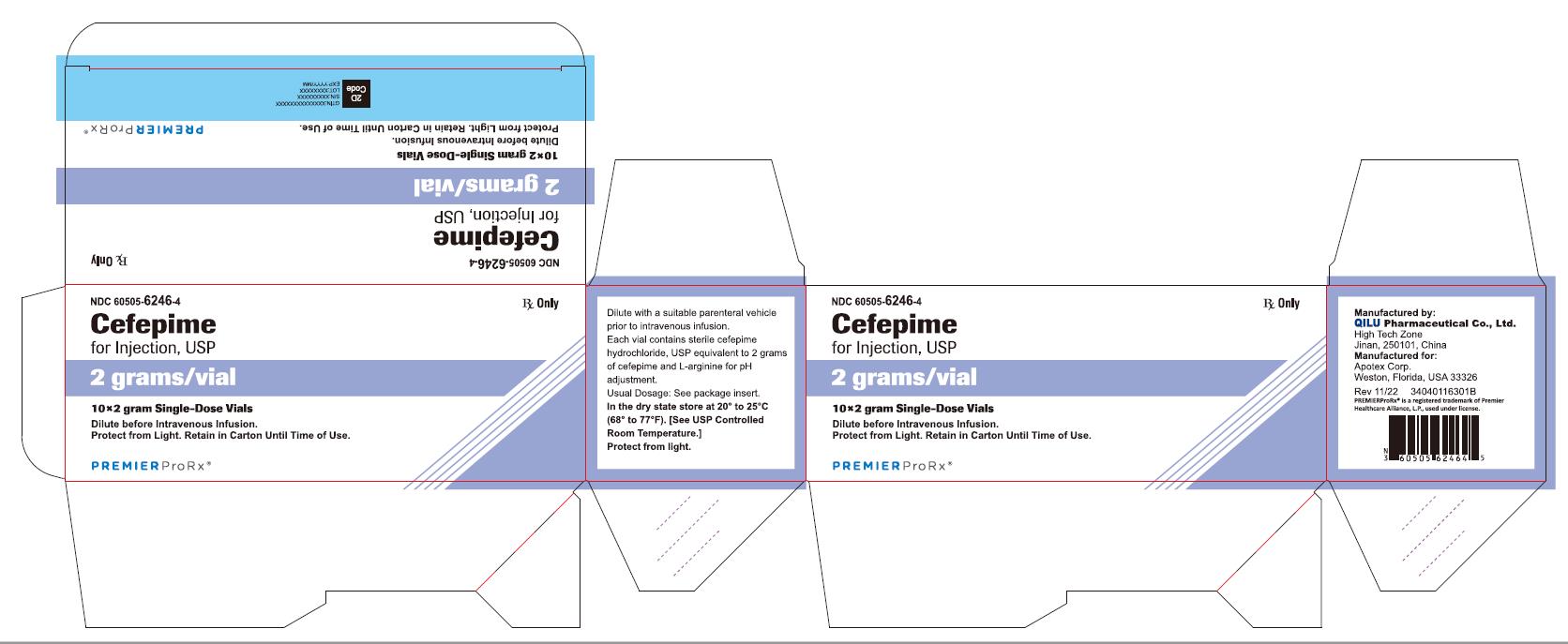
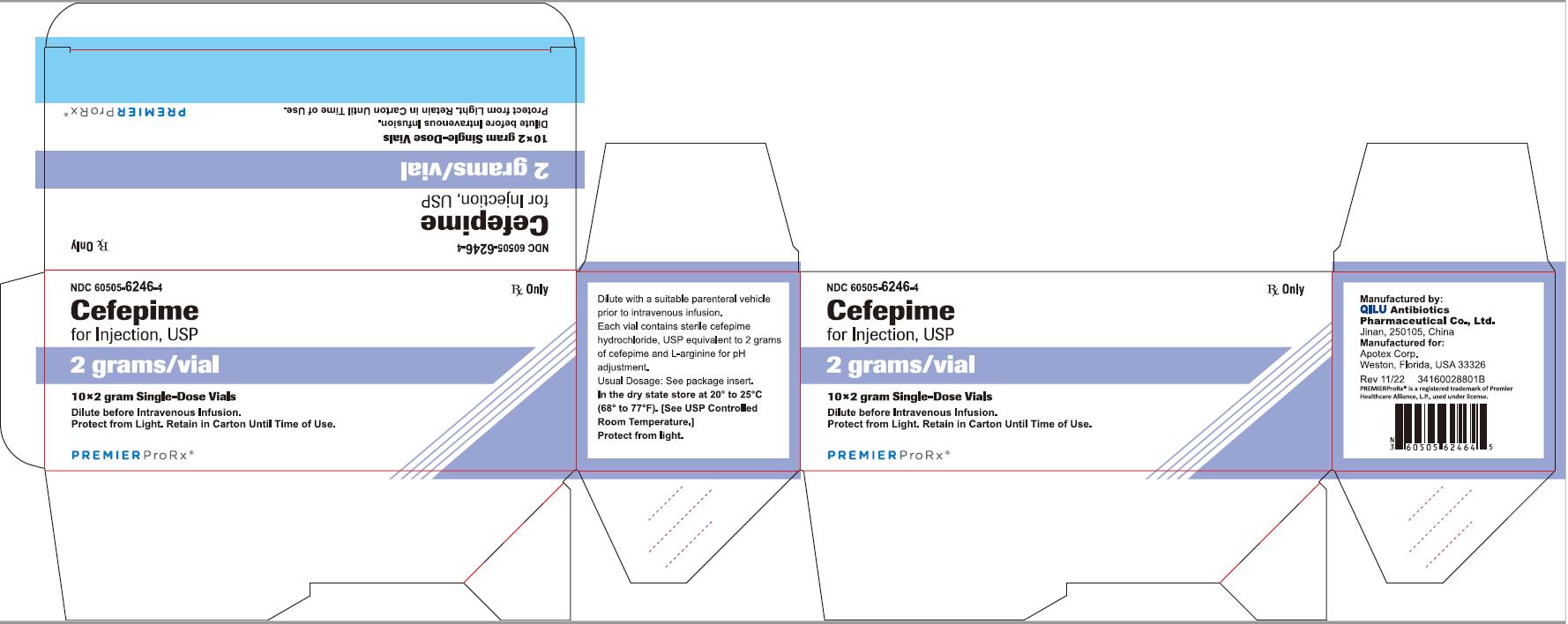
NDC 60505-6246-4 Rx Only
Cefepime
for Injection, USP
2 grams per vial
10*2 gram Single-Dose Vials
Dilute before Intravenous Infusion.
Protect from Light. Retain in Carton Until Time of Use.
P R E M I E R P r o R x ®