NDC Code(s) : 60635-107-01, 60635-111-01, 60635-118-01, 60635-133-01, 60635-123-01
Packager : Biocompatibles, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Varithenapolidocanol KIT | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Varithenapolidocanol KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Varithenapolidocanol KIT | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Varithenapolidocanol KIT | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Varithenapolidocanol KIT | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Biocompatibles, Inc.(024194234) |
| REGISTRANT - Provensis Ltd(236996703) |
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Varithena Bi-Canister - NDC 60635-107-01

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Varithena Pouch Label - NDC 60635-107-01

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Varithena Canister Label - NDC 60635-007-01

PRINCIPAL DISPLAY PANEL
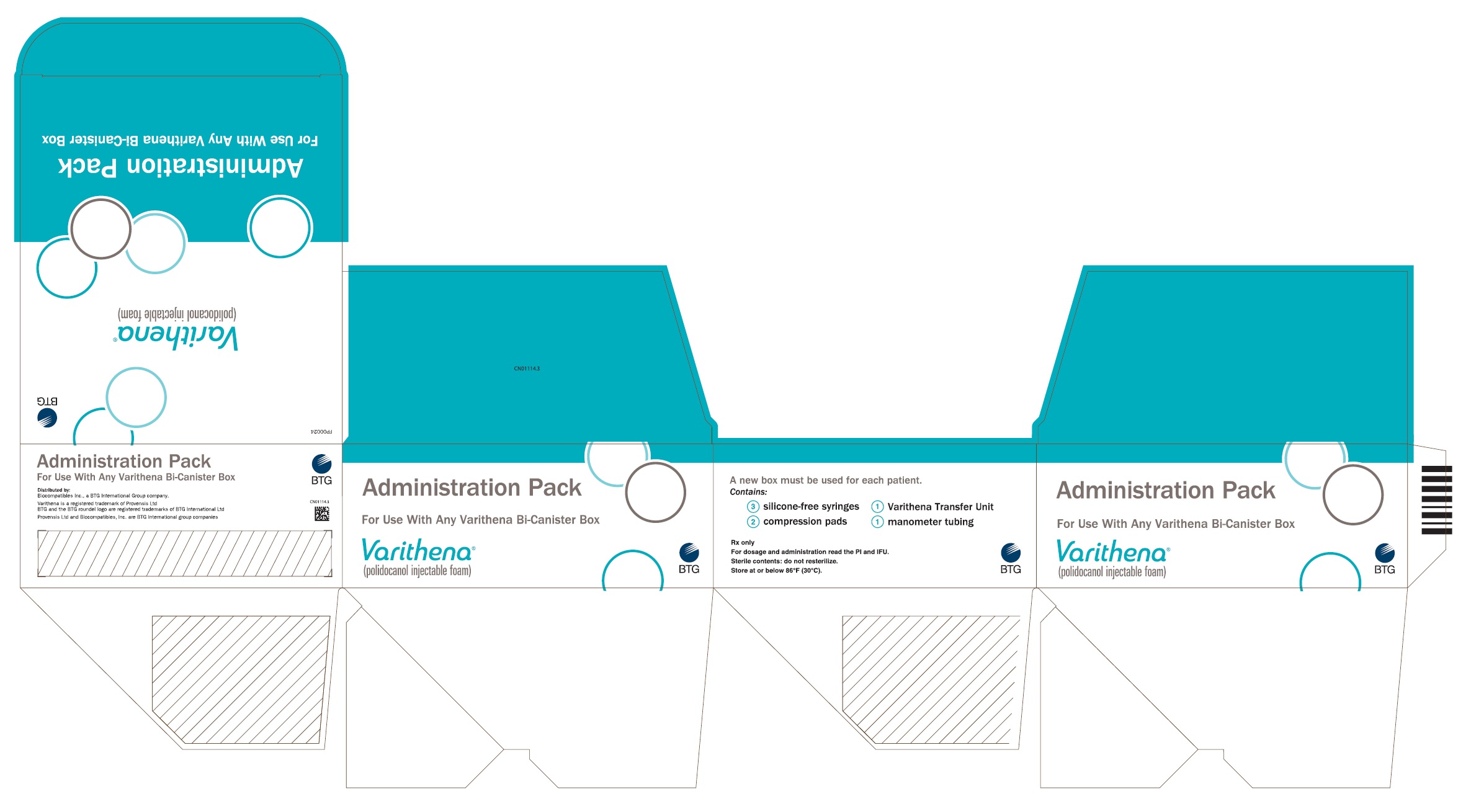
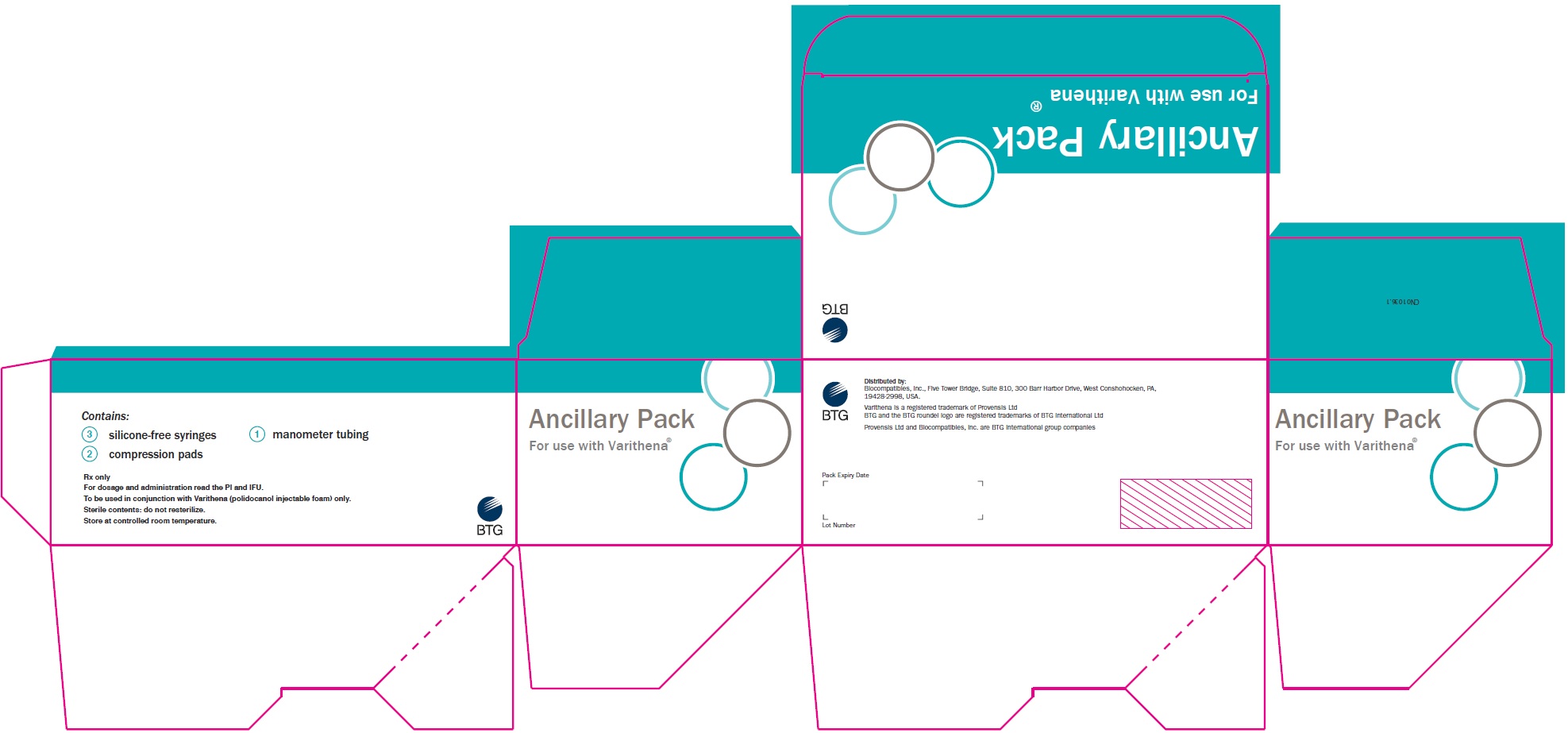
Principal Display Panel - Varithena Ancillary Pack
PRINCIPAL DISPLAY PANEL
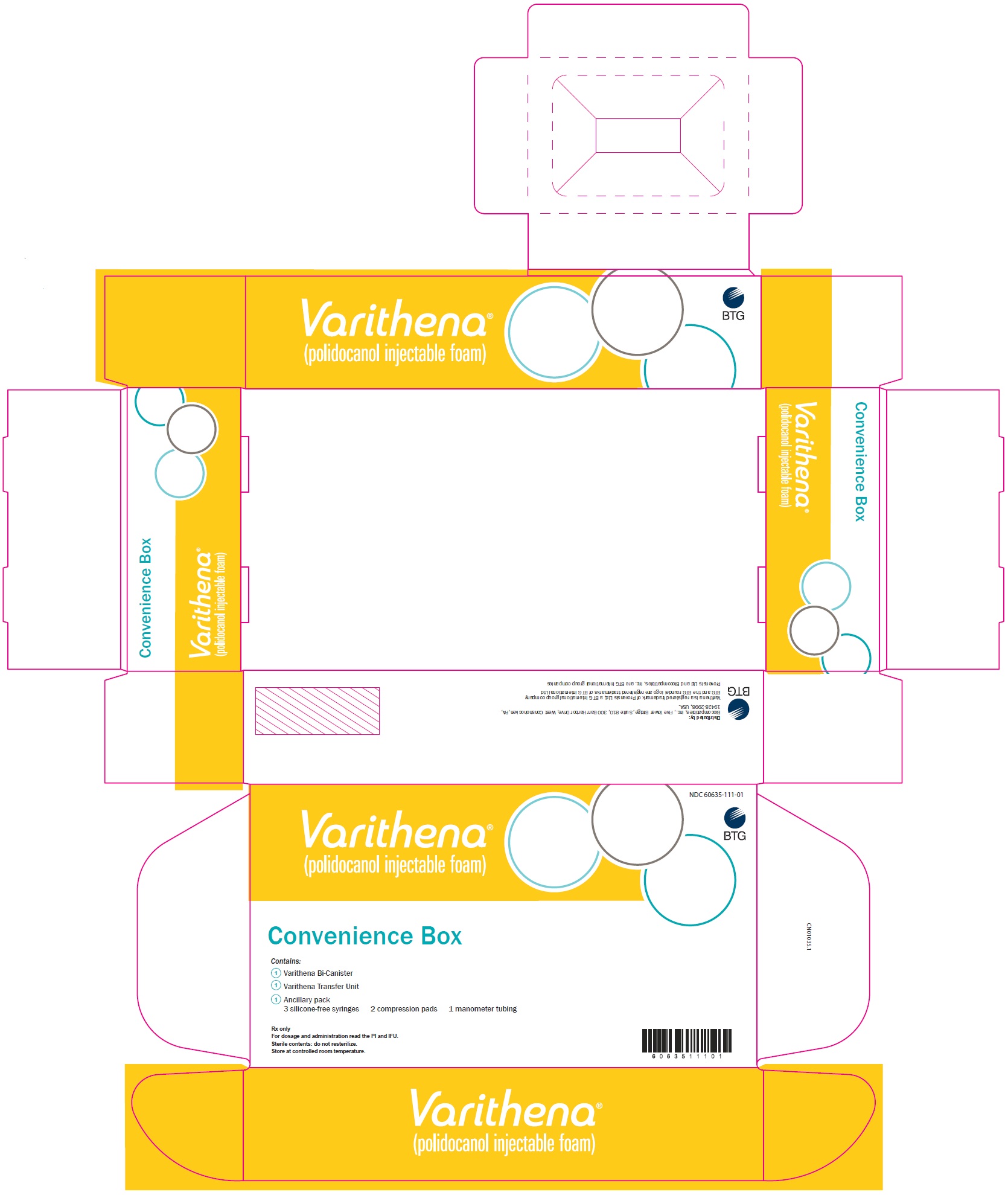
Principal Display Panel - Varithena Convenience Box Carton - NDC 60635-111-01
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Varithena Canister Label - NDC 60635-018-01

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Varithena Pouch Label - NDC 60635-118-01

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Varithena Bi-Canister Box - NDC 60635-118-01

PRINCIPAL DISPLAY PANEL
Principal Display Panel - Varithena Universal Administration Pack