NDC Code(s) : 60687-140-11, 60687-140-21, 60687-151-95, 60687-151-25
Packager : American Health Packaging
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Methylphenidate Hydrochloride Methylphenidate Hydrochloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Methylphenidate Hydrochloride Methylphenidate Hydrochloride CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL

NDC 60687-140-21
Once Daily
Methylphenidate HCl
Extended-Release Capsules (CD)
CII
10 mg
30 Capsules (3 x 10)
PHARMACIST: Dispense with the accompanying
Medication Guide to each patient.
Each Extended-Release Capsule Contains:
Methylphenidate Hydrochloride, USP ……….. 10 mg
Usual Dosage: See package insert for full prescribing
information.
Do not chew or crush the capsules or the beads inside
the capsule.
Store at 20° to 25°C (68° to 77°F); excursions permitted
between 15° to 30°C (59° to 86°F) [see USP Controlled
Room Temperature].
DEA Order Form Required.
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.
Rx Only
The drug product contained in this package is
from NDC # 0093-5295, Teva Pharmaceuticals
USA, Inc.
Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217
714021
0414021/0116
PRINCIPAL DISPLAY PANEL
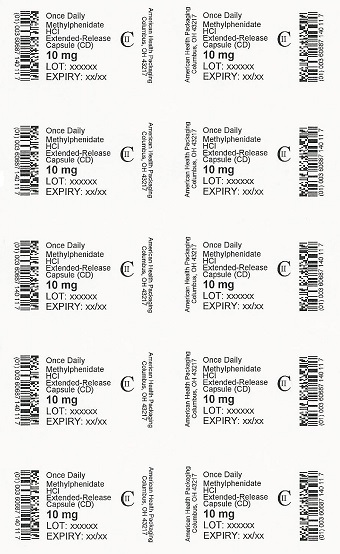
Once Daily
Methylphenidate
HCl
Extended-Release
Capsule (CD)
CII
10 mg
PRINCIPAL DISPLAY PANEL

NDC 60687-151-25
Once Daily
Methylphenidate HCl
Extended-Release
Capsules (CD)
CII
30 mg
30 Capsules (5 x 6)
PHARMACIST: Dispense with the accompanying
Medication Guide to each patient.
Each Extended-Release Capsule Contains:
Methylphenidate hydrochloride, USP ……….. 30 mg
Usual Dosage: See package insert for full
prescribing information.
Do not chew or crush the capsules or the beads
inside the capsule.
Store at 20° to 25°C (68° to 77°F); excursions
permitted between 15° to 30°C (59° to 86°F) [see
USP Controlled Room Temperature].
DEA Order Form Required.
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is
torn or broken.
Rx Only
The drug product contained in this
package is from NDC # 0093-5297,
Teva Pharmaceuticals USA, Inc.
Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217
715125
0415125/1115
PRINCIPAL DISPLAY PANEL
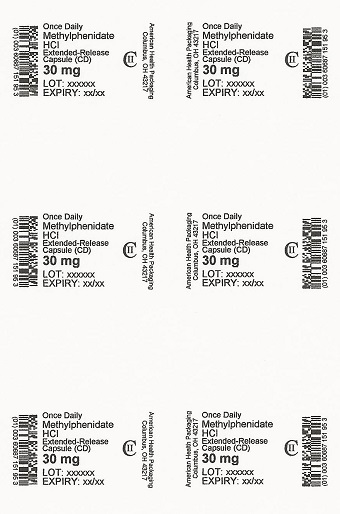
Once Daily
Methylphenidate
HCl
Extended-Release
Capsule (CD)
CII
30 mg









