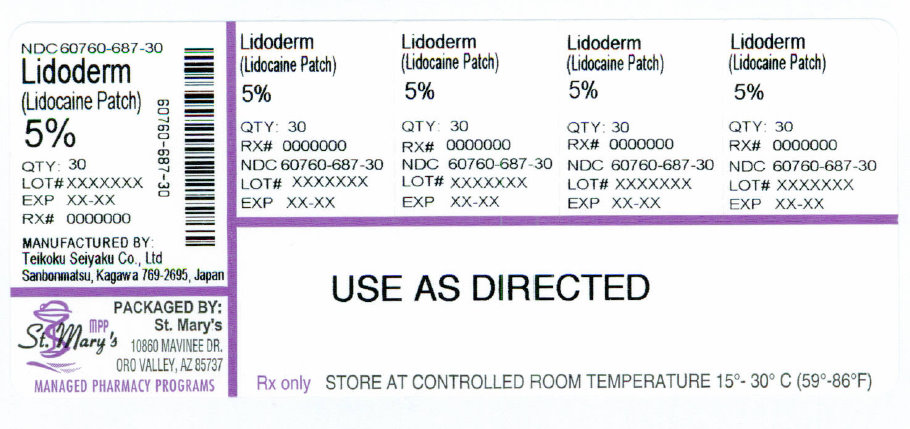
NDC Code(s) : 60760-687-30
Packager : St Marys Medical Park Pharmacy
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lidoderm 5 LIDOCAINE LIQUID | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Cut along dotted line
NDC 63481-687-01
ENDO PHARMACEUTICALS
Lidoderm LIDOCAINA PATCH 5%
Rx only
Each adhesive patch contains:
Lidocaine . . . . . . . . 700 mg ( 50 mg per gram adhesive) in an aqueous base.
Methylparaben and propylparaben as preservatives.
DOSAGE: For dosage and full prescribing information, read accompanying product information.
1 PATCH (10 cm x 14 cm)
Store at 25°C (77°F) ; excursions permitted to 15°-30°C (59°-86°F).
WARNING: Keep used and unused patches out of the reach of children, pets and others.
Manufactured for: Endo Pharmaceuticals Inc., Chadds Ford, PA 19317
Manufactured by: Teikoku Seiyaku Co., Ltd., Sanbonmatsu, Kagawa 769-2695, Japan
PRINCIPAL DISPLAY PANEL
DIRECTIONS FOR USE
Do not store patch outside the sealed envelope.
Cut the envelope along the dotted line. Patches may be cut into smaller sizes with scissors prior to removal of the release liner. Safely discard the remaining unused pieces of cut patches where children and pets cannot get to them.
Remove the transparent release liner (clear plastic backing) before applications of patch to the skin.
Apply immediately after removal from the protective envelope.
Apply up to three (3) LIDODERM patches at one time to cover the most painful area. Apply patches only once for up to 12 hours in a 24 hours period (12 hours on and 12 hours off). Remove patch if irritation occurs.
Fold used patches so that the adhesive side sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get to them.
5 63481 - 687 -017
90001/YD
LOT:
EXP:
PRINCIPAL DISPLAY PANEL
Lidodern
LIDOCAINE PATCH 5%
NDC 63481-687-01
ENDO PHARMACEUTICALS
Lidoderm LIDOCAINA PATCH 5%
Rx only
Each adhesive patch contains:
Lidocaine . . . . . . . . 700 mg ( 50 mg per gram adhesive) in an aqueous base.
Methylparaben and propylparaben as preservatives.
Usual Dosage: See package insert for complete prescribing information.
30 PATCHES
30 Envelopes
Containing 1 Patch Each
Store at 25°C (77°F) ; excursions permitted to 15°-30°C (59°-86°F).
WARNING: Keep used and unused patches out of the reach of children, pets and others.
Manufactured for: Endo Pharmaceuticals Inc., Chadds Ford, PA 19317
Manufactured by: Teikoku Seiyaku Co., Ltd., Sanbonmatsu, Kagawa 769-2695, Japan
Lidodern
LIDOCAINE PATCH 5%
DIRECTIONS FOR USE
Do not store patch outside the sealed envelope.
Cut the envelope along the dotted line. Patches may be cut into smaller sizes with scissors prior to removal of the release liner. Safely discard the remaining unused pieces of cut patches where children and pets cannot get to them.
Remove the transparent release liner (clear plastic backing) before applications of patch to the skin.
Apply immediately after removal from the protective envelope.
Apply up to three (3) LIDODERM patches at one time to cover the most painful area. Apply patches only once for up to 12 hours in a 24 hours period (12 hours on and 12 hours off). Remove patch if irritation occurs.
Fold used patches so that the adhesive side sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get to them.
8777/YD
5 63481 - 687 -017
ENDO PHARMACEUTICALS
Lidoderm
LIDOCAINA PATCH 5%
PRINCIPAL DISPLAY PANEL