NDC Code(s) : 60793-700-01, 60793-700-10, 60793-701-02, 60793-701-10, 60793-702-04, 60793-702-10
Packager : Pfizer Laboratories Div Pfizer Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BICILLIN L-Apenicillin G benzathine INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| BICILLIN L-Apenicillin G benzathine INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| BICILLIN L-Apenicillin G benzathine INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Pfizer Laboratories Div Pfizer Inc(134489525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| King Pharmaceuticals LLC | 962691478 | ANALYSIS(60793-700, 60793-701, 60793-702), MANUFACTURE(60793-700, 60793-701, 60793-702), PACK(60793-700, 60793-701, 60793-702), LABEL(60793-700, 60793-701, 60793-702) | |
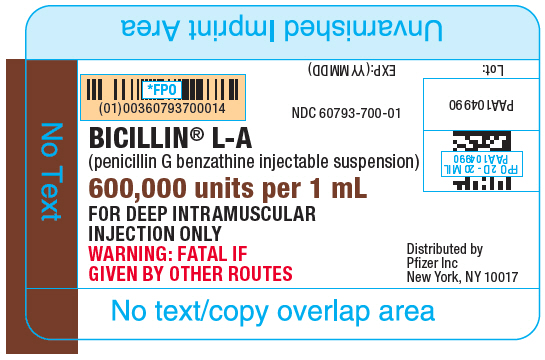
PRINCIPAL DISPLAY PANEL
NDC 60793-700-01
BICILLIN® L-A
(penicillin G benzathine injectable suspension)
600,000 units per 1 mL
FOR DEEP INTRAMUSCULAR
INJECTION ONLY
WARNING: FATAL IF
GIVEN BY OTHER ROUTES
Distributed by
Pfizer Inc
New York, NY 10017
PRINCIPAL DISPLAY PANEL
NDC 60793-700-10
Contains 10 of NDC 60793-700-01
Ten Syringes (1 mL size)
Bicillin® L-A
(penicillin G benzathine injectable suspension)
600,000 units per 1 mL
FOR PEDIATRIC USE
FOR DEEP INTRAMUSCULAR INJECTION ONLY
WARNING: FATAL IF GIVEN BY OTHER ROUTES
BEFORE INJECTING, SEE PACKAGE INSERT FOR ADMINISTRATION INSTRUCTIONS.
Pfizer Injectables
Rx only

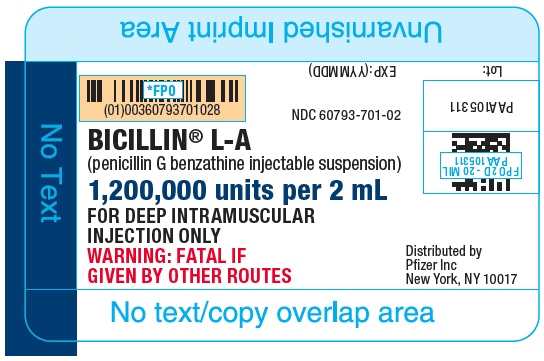
PRINCIPAL DISPLAY PANEL
NDC 60793-701-02
BICILLIN® L-A
(penicillin G benzathine injectable suspension)
1,200,000 units per 2 mL
-
FOR DEEP INTRAMUSCULAR
INJECTION ONLY
WARNING: FATAL IF
GIVEN BY OTHER ROUTES
Distributed by
Pfizer Inc
New York, NY 10017
PRINCIPAL DISPLAY PANEL
NDC 60793-701-10
Contains 10 of NDC 60793-701-02
Ten Syringes (2 mL size)
Bicillin® L-A
(penicillin G benzathine injectable suspension)
1,200,000 units per 2 mL
FOR DEEP INTRAMUSCULAR INJECTION ONLY
WARNING: FATAL IF GIVEN BY OTHER ROUTES
BEFORE INJECTING, SEE PACKAGE INSERT FOR ADMINISTRATION INSTRUCTIONS.
Pfizer Injectables
Rx only

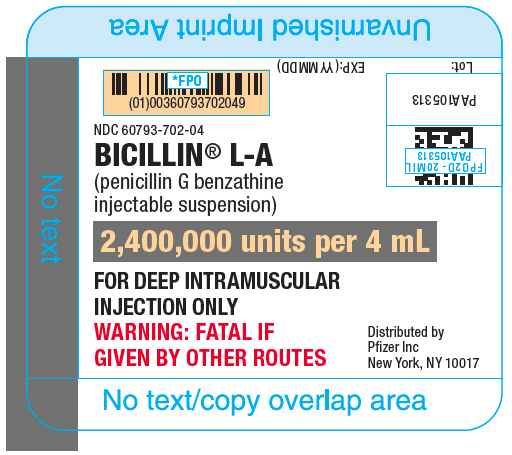
PRINCIPAL DISPLAY PANEL
NDC 60793-702-04
BICILLIN® L-A
(penicillin G benzathine
injectable suspension)
2,400,000 units per 4 mL
FOR DEEP INTRAMUSCULAR
INJECTION ONLY
WARNING: FATAL IF
GIVEN BY OTHER ROUTES
Distributed by
Pfizer Inc
New York, NY 10017
PRINCIPAL DISPLAY PANEL
NDC 60793-702-10
Contains 10 of NDC 60793-702-04
Ten Syringes (4 mL size)
Bicillin® L-A
(penicillin G benzathine injectable suspension)
2,400,000 units per 4 mL
disposable syringe
FOR DEEP INTRAMUSCULAR INJECTION ONLY
WARNING: FATAL IF GIVEN BY OTHER ROUTES
BEFORE INJECTING, SEE PACKAGE INSERT FOR ADMINISTRATION INSTRUCTIONS.
Pfizer Injectables
Rx only










