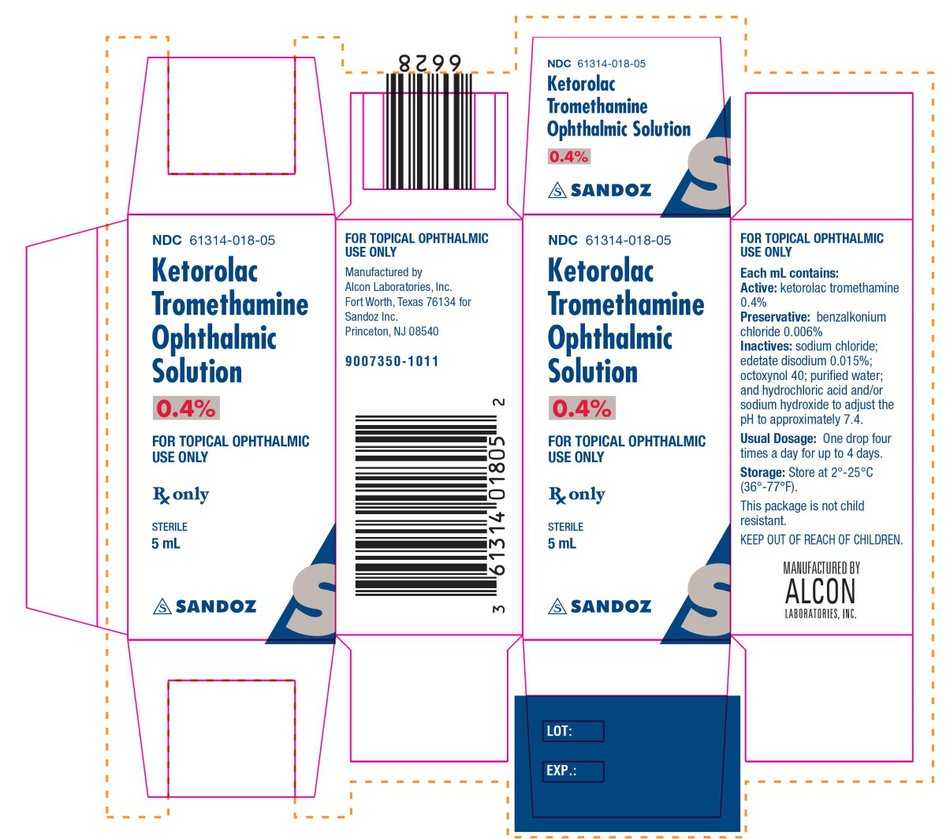
NDC Code(s) : 61314-018-05
Packager : Sandoz Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| KETOROLAC TROMETHAMINEketorolac tromethamine SOLUTION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 61314-018-05
Ketorolac Tromethamine Ophthalmic Solution, 0.4 %
FOR TOPICAL OPHTHALMIC USE ONLY
Rx only
Sterile
5 mL