NDC Code(s) : 61314-454-20, 61314-454-36, 61314-454-68, 61314-473-64, 61314-473-20, 61314-476-64, 61314-509-64, 61314-517-36, 61314-531-64
Packager : Sandoz Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| HYRIMOZadalimumab-adaz INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| HYRIMOZadalimumab-adaz INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| HYRIMOZadalimumab-adaz INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYRIMOZadalimumab-adaz INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYRIMOZadalimumab-adaz KIT | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| HYRIMOZadalimumab-adaz KIT | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Sandoz Inc(005387188) |
PRINCIPAL DISPLAY PANEL
NDC 61314-876-02
Rx only
Hyrimoz®
(adalimumab-adaz)
Injection
40 mg/0.8 mL
For Subcutaneous Use Only
2 Single-Dose Prefilled Syringes
with needle guard

PRINCIPAL DISPLAY PANEL
NDC 61314-871-02
Rx only
Hyrimoz®
(adalimumab-adaz)
Injection
40 mg/0.8 mL
For Subcutaneous Use Only
2 Single-Dose Prefilled Sensoready ® Pens

PRINCIPAL DISPLAY PANEL
NDC 61314-741-01
Hyrimoz®
(adalimumab-adaz)
Injection
10 mg/0.2 mL
Rx Only
For Subcutaneous Use Only
1Single-Dose Prefilled Syringe

PRINCIPAL DISPLAY PANEL
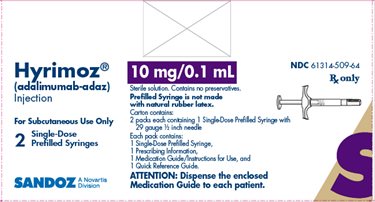
NDC 61314-509-64
Rx Only
Hyrimoz®
(adalimumab-adaz)
Injection
10 mg/0.1 mL
Rx Only
For Subcutaneous Use Only
2Single-Dose Prefilled Syringes
PRINCIPAL DISPLAY PANEL
NDC 61314-476-64
Rx Only
Hyrimoz®
(adalimumab-adaz)
Injection
20 mg/0.2 mL
For Subcutaneous Use Only
2Single-Dose Prefilled Syringes

PRINCIPAL DISPLAY PANEL
NDC 61314-850-02
Rx Only
Hyrimoz®
(adalimumab-adaz)
Injection
20 mg/0.4 mL
For Subcutaneous Use Only
2Single-Dose Prefilled Syringes with needle guard

PRINCIPAL DISPLAY PANEL
NDC 61314-473-20
Rx Only
Hyrimoz®
(adalimumab-adaz)
Injection
40 mg/0.4 mL
For Subcutaneous Use Only
2Single-Dose Prefilled Sensoready® Pens

PRINCIPAL DISPLAY PANEL
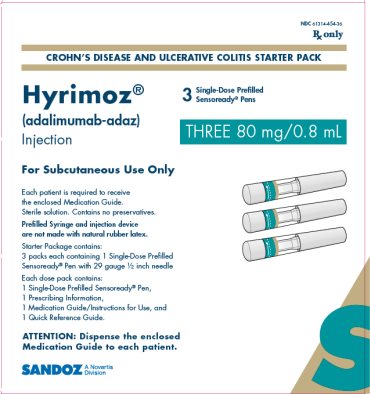
NDC 61314-454-36
Rx Only
CROHN’S DISEASE AND ULCERATIVE COLITIS STARTER PACK
Hyrimoz®
(adalimumab-adaz)
Injection
THREE 80 mg/0.8 mL
3Single-Dose Prefilled Sensoready® Pens
For Subcutaneous Use Only
Each patient is required to receive the enclosed Medication Guide.
Sterile solution. Contains no preservatives.
Prefilled Syringe and injection device are not made with natural rubber latex.
Starter Package contains:
3 packs each containing 1 Single-Dose Prefilled Sensoready ® Pen with 29 gauge ½ inch needle
Each dose pack contains:
1 Single-Dose Prefilled Sensoready ® Pen,
1 Prescribing Information,
1 Medication Guide/Instructions for Use, and
1 Quick Reference Guide.
ATTENTION: Dispense the enclosed Medication Guide to each patient.
PRINCIPAL DISPLAY PANEL
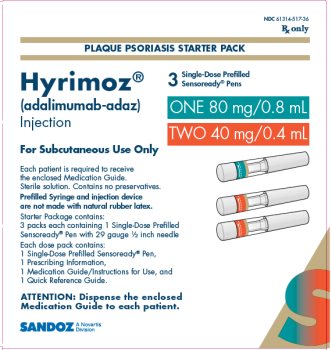
NDC 61314-517-36
Rx Only
PLAQUE PSORIASIS STARTER PACK
Hyrimoz®
(adalimumab-adaz)
Injection
ONE 80 mg/0.8 mL
TWO 40 mg/0.4 mL
For Subcutaneous Use Only
3Single-Dose Prefilled Sensoready® Pens
Each patient is required to receive the enclosed Medication Guide.
Sterile solution. Contains no preservatives.
Prefilled Syringe and injection device are not made with natural rubber latex.
Starter Package contains:
3 packs each containing 1 Single-Dose Prefilled Sensoready ® Pen with 29 gauge ½ inch needle
Each dose pack contains:
1 Single-Dose Prefilled Sensoready ® Pen,
1 Prescribing Information,
1 Medication Guide/Instructions for Use, and
1 Quick Reference Guide.
ATTENTION: Dispense the enclosed Medication Guide to each patient.
PRINCIPAL DISPLAY PANEL
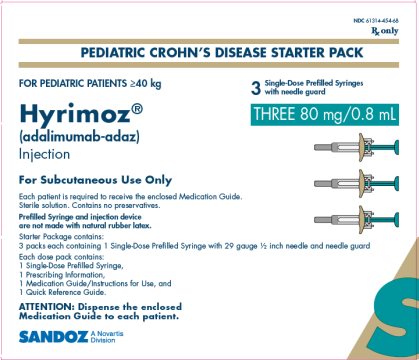
NDC 61314-454-68
Rx Only
PEDIATRIC CROHN’S DISEASE STARTER PACK
FOR PEDIATRIC PATIENTS ≥40 kg
Hyrimoz®
(adalimumab-adaz)
Injection
THREE 80 mg/0.8 mL
3Single-Dose Prefilled Syringes with needle guard
For Subcutaneous Use Only
Each patient is required to receive the enclosed Medication Guide.
Sterile solution. Contains no preservatives.
Prefilled Syringe and injection device are not made with natural rubber latex.
Starter Package contains:
3 packs each containing 1 Single-Dose Prefilled Syringe with 29 gauge ½ inch needle and needle guard
Each dose pack contains:
1 Single-Dose Prefilled Syringe,
1 Prescribing Information,
1 Medication Guide/Instructions for Use, and
1 Quick Reference Guide.
ATTENTION: Dispense the enclosed Medication Guide to each patient.
PRINCIPAL DISPLAY PANEL
NDC 61314-531-64
Rx Only
PEDIATRIC CROHN’S DISEASE STARTER PACK
FOR PEDIATRIC PATIENTS <40 kg
Hyrimoz®
(adalimumab-adaz)
Injection
ONE 80 mg/0.8 mL
ONE 40 mg/0.4 mL
2Single-Dose Prefilled Syringes with needle guard
For Subcutaneous Use Only
Each patient is required to receive the enclosed Medication Guide.
Sterile solution. Contains no preservatives.
Prefilled Syringe and injection device are not made with natural rubber latex.
Starter Package contains:
2 packs each containing 1 Single-Dose Prefilled Syringe with 29 gauge ½ inch needle and needle guard
Each dose pack contains:
1 Single-Dose Prefilled Syringe,
1 Prescribing Information,
1 Medication Guide/Instructions for Use, and
1 Quick Reference Guide.
ATTENTION: Dispense the enclosed Medication Guide to each patient.










