NDC Code(s) : 61786-951-02
Packager : REMEDYREPACK INC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| LevetiracetamLevetiracetam TABLET, FILM COATED | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
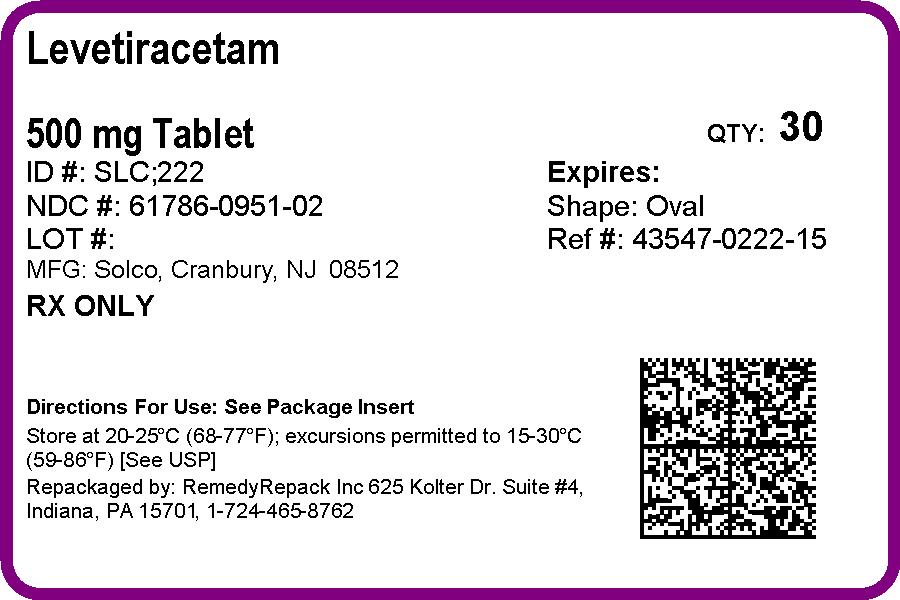
PRINCIPAL DISPLAY PANEL
DRUG: Levetiracetam
GENERIC: Levetiracetam
DOSAGE: TABLET, FILM COATED
ADMINSTRATION: ORAL
NDC: 61786-951-02
COLOR: pink
SHAPE: OVAL
SCORE: Two even pieces
SIZE: 18 mm
IMPRINT: SLC;222
PACKAGING: 30 in 1 BLISTER PACK
ACTIVE INGREDIENT(S):
- LEVETIRACETAM 500mg in 1
INACTIVE INGREDIENT(S):
- FD&C RED NO. 40
- HYPROMELLOSE 2910 (6 MPA.S)
- HYPROMELLOSE 2910 (3 MPA.S)
- HYPROMELLOSE 2910 (50 MPA.S)
- FD&C YELLOW NO. 6
- TITANIUM DIOXIDE
- POLYDEXTROSE
- POLYETHYLENE GLYCOL 800
- MAGNESIUM STEARATE
- POVIDONES
- SILICON DIOXIDE
- STARCH, CORN
- D&C RED NO. 27
- FD&C BLUE NO. 2
- TALC
- TRIACETIN