NDC Code(s) : 61787-558-15
Packager : Health Care Products
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Diaderm Antifungalundecylenic acid CREAM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
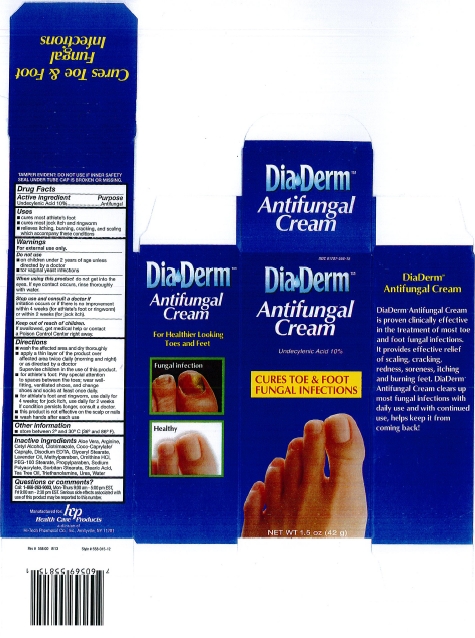
NDC 61787-558-15
Diaderm
Antifungal Cream
Undecylenic Acid 10%
CURES TOE & FOOT FUNGAL INFECTIONS
NET WT 1.5 oz (42 g)









