NDC Code(s) : 62168-0584-4, 62168-0584-5, 62168-0584-6
Packager : Lead Chemical Co. Ltd.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AspercremeLidocaine PATCH | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Lead Chemical Co. Ltd.(693727091) |
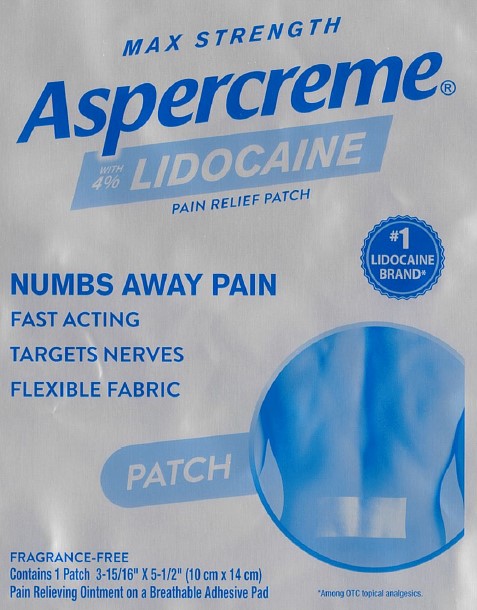
PRINCIPAL DISPLAY PANEL
MAX STRENGTH
Aspercreme®with 4% LIDOCAINE
PAIN RELIEF PATCH
NUMBS AWAY PAINFAST ACTING
TARGETS NERVES
FLEXIBLE FABRIC
#1 LIDOCAINE BRAND*
PATCH
FRAGRANCE-FREE
Contains 1 Patch 3-15/16” x 5-1/2” (10 cm x 14 cm)
Pain Relieving Ointment on a Breathable Adhesive Pad
*Among OTC topical analgesics.
Dist. by Chattem, Inc., a Sanofi Company
P.O. Box 2219, Chattanooga, TN
37409-0219 USA
©2022
www.aspercreme.com Made in Japan
Label
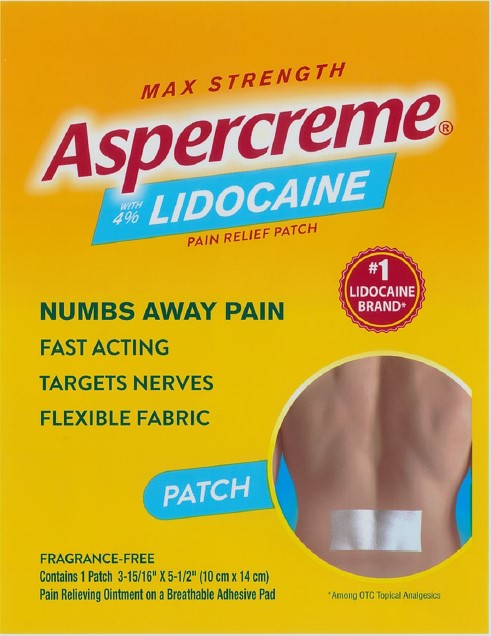
PRINCIPAL DISPLAY PANEL
MAX STRENGTH
Aspercreme®with 4% LIDOCAINE
PAIN RELIEF PATCH
NUMBS AWAY PAINFAST ACTING
TARGETS NERVES
FLEXIBLE FABRIC
#1 LIDOCAINE BRAND*
PATCH
FRAGRANCE-FREE
Contains 1 Patch 3-15/16” x 5-1/2” (10 cm x 14 cm)
Pain Relieving Ointment on a Breathable Adhesive Pad
*Among OTC topical analgesics
CHATTEM®
A SANOFI COMPANY
Dist. by Chattem, Inc., a Sanofi Company
P.O. Box 2219, Chattanooga, TN
37409-0219 USA ©2020
www.aspercreme.com
Made in Japan
Label









