NDC Code(s) : 62332-171-30, 62332-171-60, 62332-171-91, 62332-171-10, 62332-172-30, 62332-172-60, 62332-172-91, 62332-172-10, 62332-173-30, 62332-173-60, 62332-173-91, 62332-173-10, 62332-174-30, 62332-174-60, 62332-174-71, 62332-174-10
Packager : Alembic Pharmaceuticals Inc.
Category : Human Prescription Drug Label
DEA Schedule : CV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lacosamide lacosamide TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Lacosamide lacosamide TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Lacosamide lacosamide TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Lacosamide lacosamide TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Alembic Pharmaceuticals Inc.(079288842) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alembic Pharmaceuticals Limited | 650574671 | MANUFACTURE(62332-171, 62332-172, 62332-173, 62332-174) | |
PRINCIPAL DISPLAY PANEL
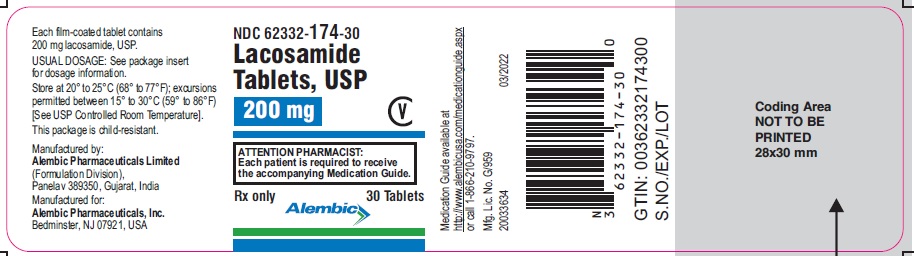
NDC 62332-171-30
Lacosamide Tablets,USP
50 mg
CV
ATTENTION PHARMACIST:
Each patient is required to receive
the accompanying Medication Guide.
Rx only
30 Tablets
Alembic
PRINCIPAL DISPLAY PANEL
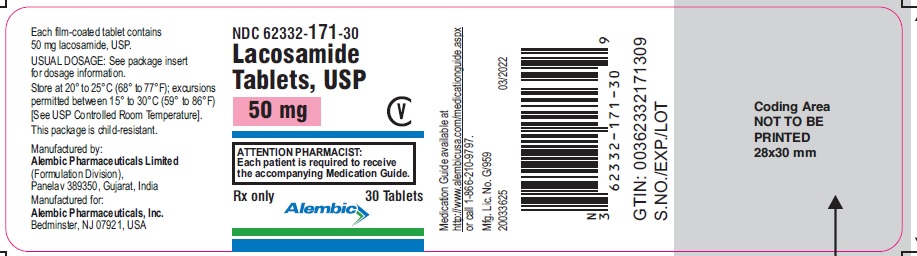
NDC 62332-172-30
Lacosamide Tablets, USP
100 mg
CV
ATTENTION PHARMACIST:
Each patient is required to receive
the accompanying Medication Guide.
Rx only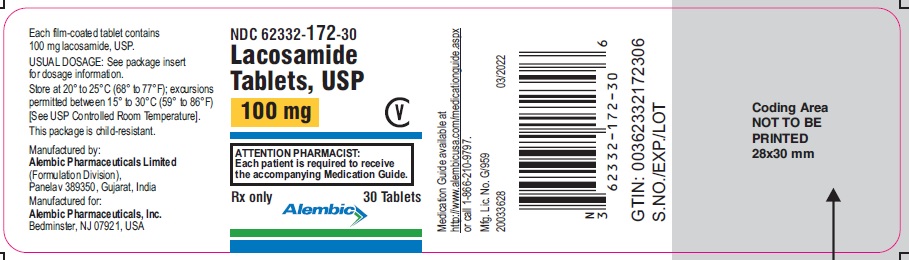
Alembic
PRINCIPAL DISPLAY PANEL
NDC 62332-173-30
Lacosamide Tablets, USP
150 mg
CV
ATTENTION PHARMACIST:
Each patient is required to receive
the accompanying Medication Guide.
Rx only
30 Tablets
Alembic
PRINCIPAL DISPLAY PANEL
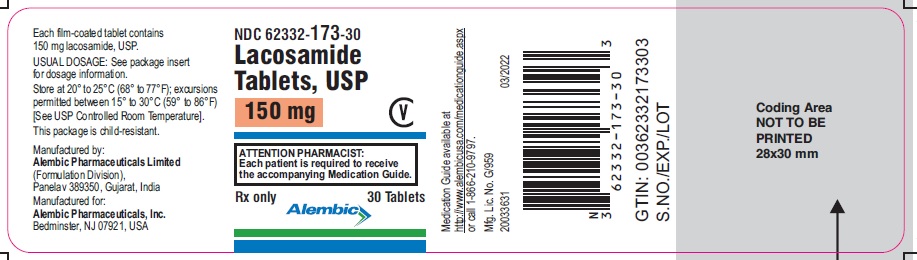
NDC 62332-174-30
Lacosamide Tablets, USP
200 mg
CV
ATTENTION PHARMACIST:
Each patient is required to receive
the accompanying Medication Guide.
Rx only
30 Tablets
Alembic