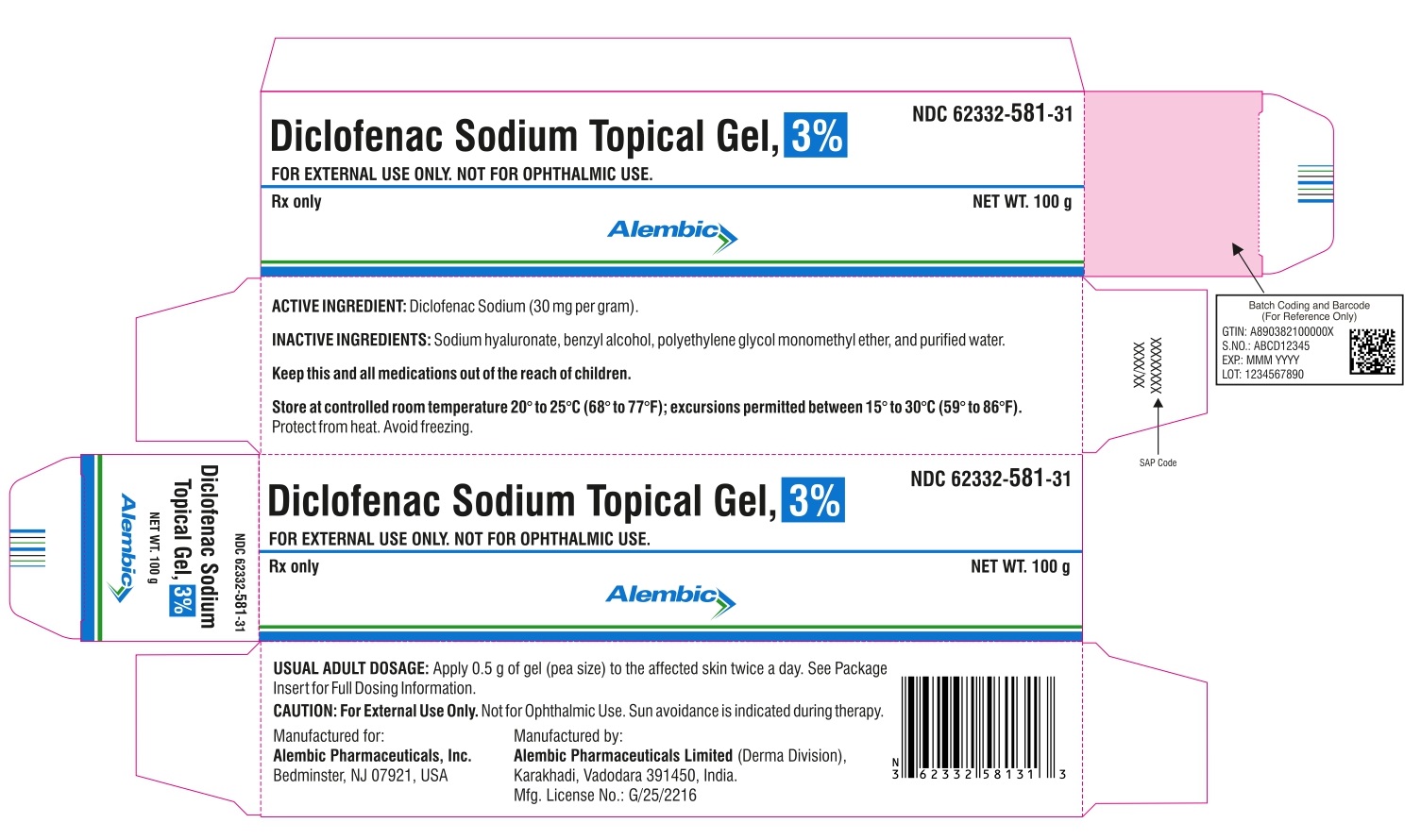
NDC Code(s) : 62332-581-31
Packager : Alembic Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Diclofenac Sodium Diclofenac Sodium Topical GEL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Alembic Pharmaceuticals Inc.(079288842) |
| REGISTRANT - Alembic Pharmaceuticals Limited(650574663) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alembic Pharmaceuticals Limited | 871411532 | MANUFACTURE(62332-581), ANALYSIS(62332-581) | |
PRINCIPAL DISPLAY PANEL
NDC 62332-581-31
Net Wt. 100 g
Rx Only
Diclofenac Sodium Topical Gel, 3%