NDC Code(s) : 62756-091-40
Packager : Sun Pharmaceutical Industries, Inc.
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| MEDROXYPROGESTERONE ACETATE MEDROXYPROGESTERONE ACETATE INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Sun Pharmaceutical Industries, Inc.(146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sun Pharmaceutical Industries Limited | 725959238 | ANALYSIS(62756-091), MANUFACTURE(62756-091) | |
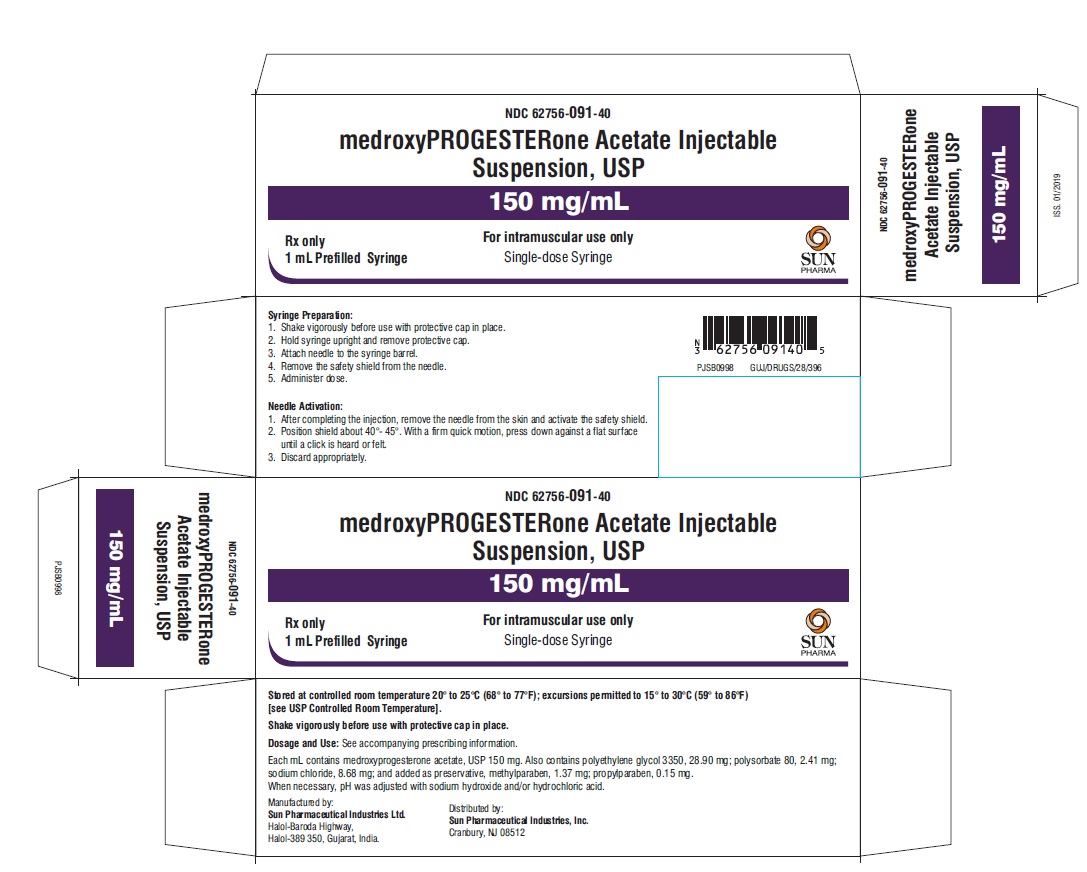
PRINCIPAL DISPLAY PANEL
NDC 62756-091-40
medroxyPROGESTERone Acetate Injectable Suspension, USP
150 mg/mL
For intramuscular use only
Single-dose Syringe
Rx only
1 mL Prefilled Syringe
Sun Pharma
PRINCIPAL DISPLAY PANEL
NDC 62756-091-40
Rx only
medroxyPROGESTERone Acetate Injectable Suspension, USP
150 mg/mL
1 mL Single-dose Syringe
Intramuscular use only
Shake vigorously before use










