NDC Code(s) : 62756-700-83, 62756-700-88, 62756-700-08, 62756-700-18, 62756-700-64, 62756-700-84, 62756-701-83, 62756-701-88, 62756-701-08, 62756-701-18, 62756-701-64, 62756-701-84, 62756-702-83, 62756-702-88, 62756-702-08, 62756-702-18, 62756-702-64, 62756-702-84, 62756-633-83, 62756-633-88, 62756-633-08, 62756-633-18, 62756-633-84, 62756-634-83, 62756-634-88, 62756-634-08, 62756-634-18, 62756-634-84
Packager : Sun Pharmaceutical Industries Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Risperidone Risperidone TABLET, ORALLY DISINTEGRATING | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Risperidone Risperidone TABLET, ORALLY DISINTEGRATING | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Risperidone Risperidone TABLET, ORALLY DISINTEGRATING | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Risperidone Risperidone TABLET, ORALLY DISINTEGRATING | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Risperidone Risperidone TABLET, ORALLY DISINTEGRATING | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
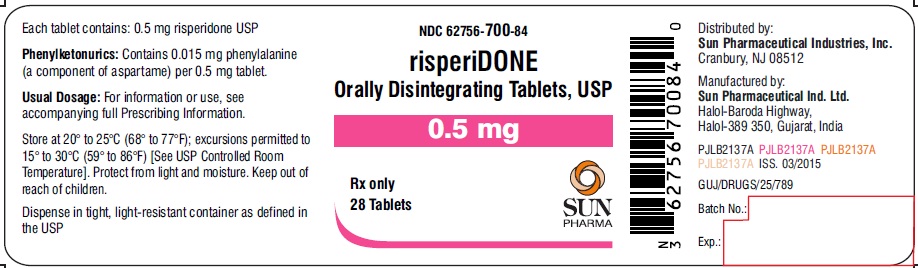
PRINCIPAL DISPLAY PANEL
NDC 62756-700-84
risperiDONE Orally Disintegrating Tablets, USP
0.5 mg
Rx only
28 Tablets
SUN PHARMA
PRINCIPAL DISPLAY PANEL
NDC 62756-701-84
risperiDONE Orally Disintegrating Tablets, USP
1 mg
Rx only
28 Tablets
SUN PHARMA

PRINCIPAL DISPLAY PANEL
NDC 62756-702-84
risperiDONE Orally Disintegrating Tablets, USP
2 mg
Rx only
28 Tablets
SUN PHARMA

PRINCIPAL DISPLAY PANEL
NDC 62756-633-84
risperiDONE Orally Disintegrating Tablets, USP
3 mg
Rx only
28 Tablets
SUN PHARMA

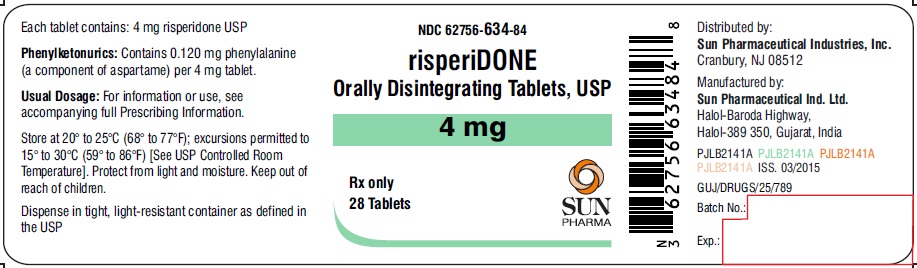
PRINCIPAL DISPLAY PANEL
NDC 62756-634-84
risperiDONE Orally Disintegrating Tablets, USP
4 mg
Rx only
28 Tablets
SUN PHARMA