NDC Code(s) : 62856-582-52, 62856-582-04, 62856-583-14, 62856-583-52, 62856-584-46
Packager : Eisai Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Banzelrufinamide TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Banzelrufinamide TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Banzelrufinamide SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Eisai Inc.(189246791) |
| REGISTRANT - Eisai Co., Ltd.(695153262) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Ajinomoto Omnichem | 400344442 | api manufacture(62856-582, 62856-583, 62856-584) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Bushu Pharmaceuticals, Ltd. | 692386648 | manufacture(62856-582, 62856-583), pack(62856-582, 62856-583) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Catalent Pharma Solutions, LLC | 014167995 | analysis(62856-582, 62856-583) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Delpharm Huningue | 262639390 | analysis(62856-584), manufacture(62856-584), pack(62856-584) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharma Packaging Solutions, LLC dba Tjoapack, LLC | 928861723 | label(62856-582, 62856-583, 62856-584), pack(62856-582, 62856-583, 62856-584) | |
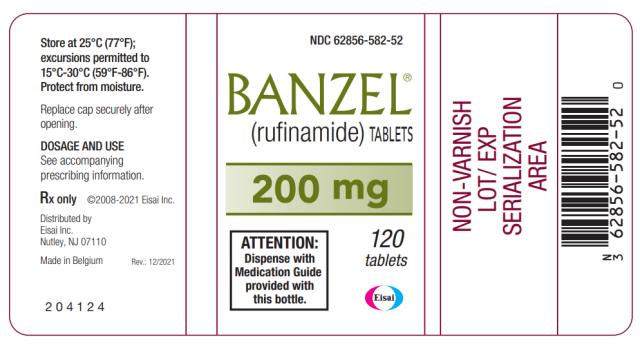
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 62856-582-52
BANZEL
®
(rufinamide) TABLETS
200 mg
120 tablets
PRINCIPAL DISPLAY PANEL
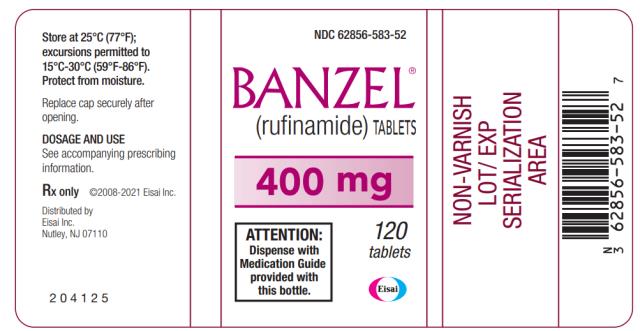
PRINCIPAL DISPLAY PANEL
NDC 62856-583
-52
BANZEL
®
(rufinamide) TABLETS
400 mg
120 tablets
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 62856-584-46
BANZEL
®
(rufinamide)
ORAL SUSPENSION
40 mg/mL
460 mL

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 62856-584-46
BANZEL
®
(rufinamide)
ORAL SUSPENSION
40 mg/mL
460 mL










