NDC Code(s) : 63323-147-10
Packager : Fresenius Kabi USA, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| PeditraceZINC, COPPER, MANGANESE, SELENIUM, FLUORINE, and IODINE INJECTION, SOLUTION | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
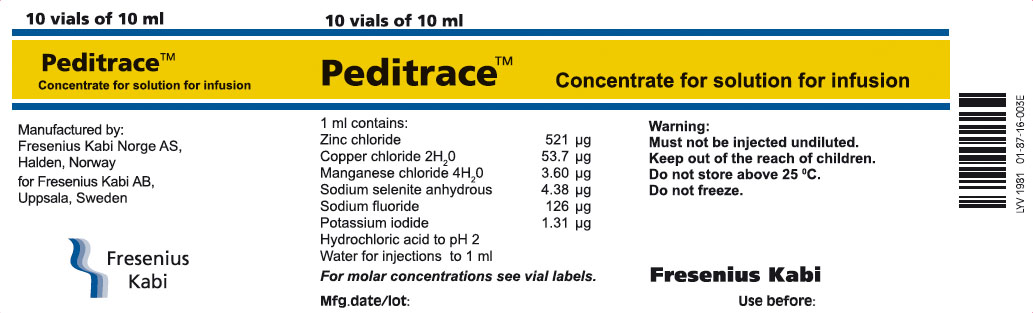
PRINCIPAL DISPLAY PANEL
Peditrace™
10 ml
Concentrate for solution for infusion

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Peditrace 10 mL Vial Carton Panel
10 vials of 10 ml
Peditrace™
Concentrate for solution for infusion