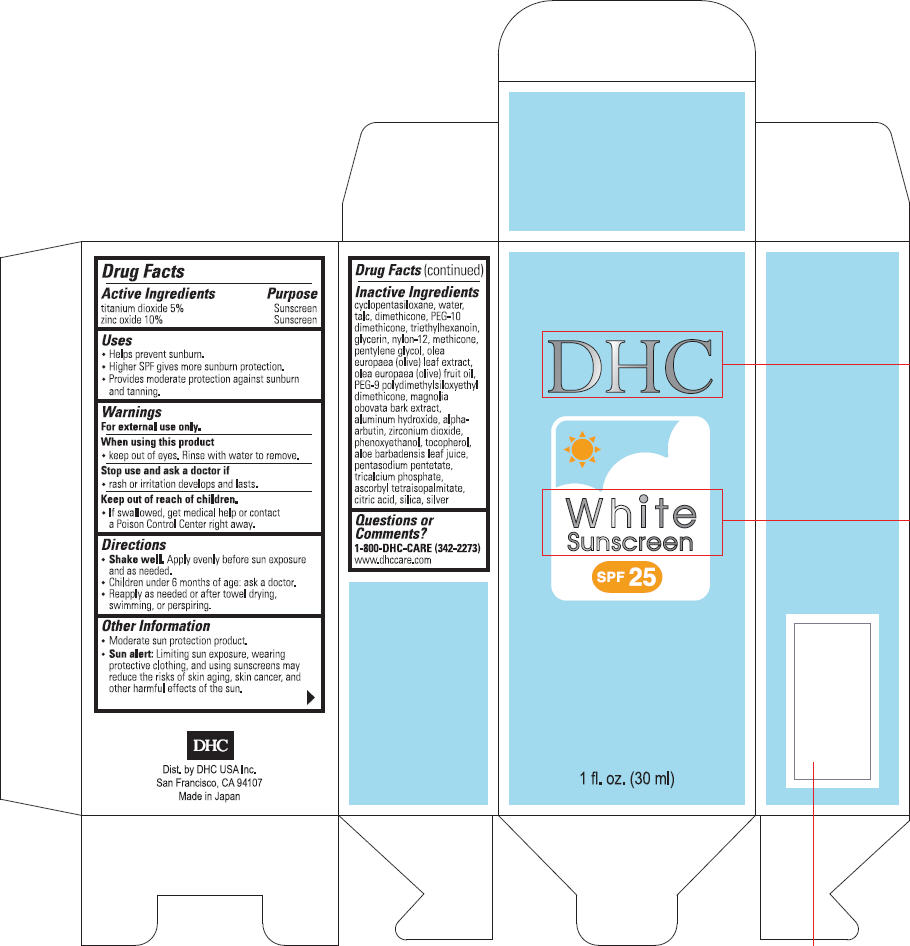
NDC Code(s) : 63433-451-51, 63433-451-79
Packager : DHC USA Incorporated
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DHC White SunscreenTitanium Dioxide and Zinc Oxide CREAM | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
DHC
White
Sunscreen
SPF 25
1 fl. oz. (30 ml)