NDC Code(s) : 63459-348-04
Packager : Teva Pharmaceuticals USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Bendekabendamustine hydrochloride INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Teva Pharmaceuticals USA, Inc.(183236314) |
PRINCIPAL DISPLAY PANEL
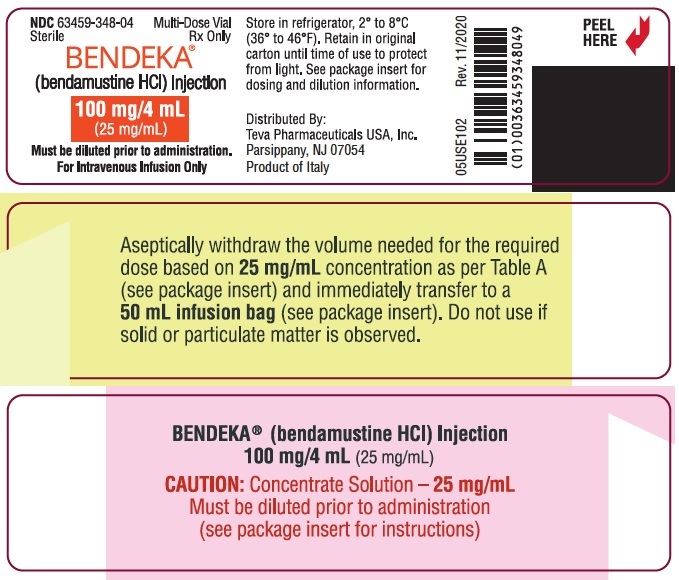
NDC 63459-348-04
Sterile
Rx Only
Multi-Dose Vial
BENDEKA™
(bendamustine HCl) Injection
100 mg/4 mL
(25 mg/mL)
For Intravenous Infusion Only
Must be diluted prior to administration.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Product of India
GUJ/DRUGS/G/28/1304
PRINCIPAL DISPLAY PANEL
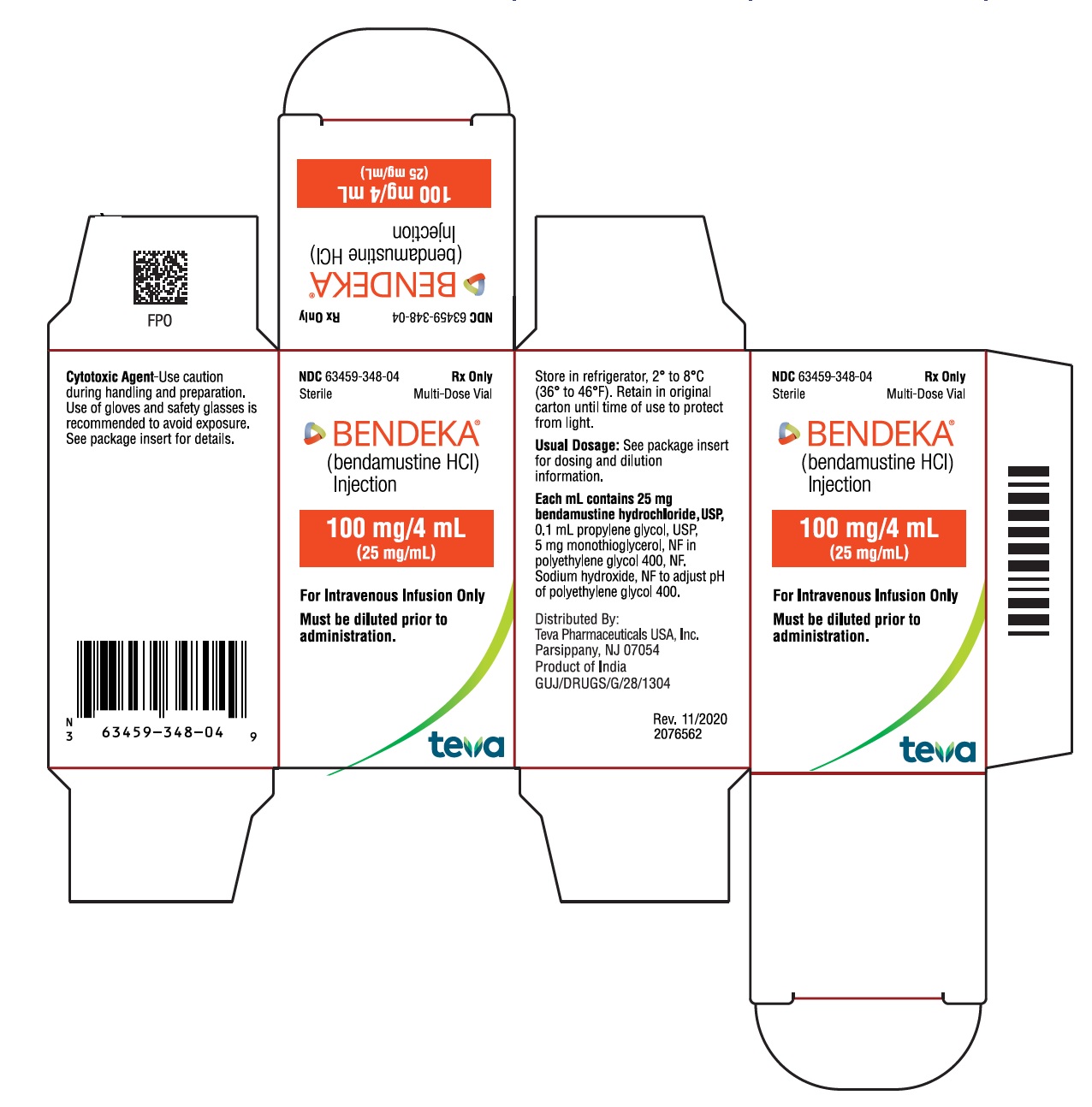
NDC 63459-348-04
Sterile
Multi-Dose Vial
Rx Only
BENDEKA™
(bendamustine HCl) Injection
100 mg/4 mL
(25 mg/mL)
Must be diluted prior to administration.
For Intravenous Infusion Only
Store in refrigerator, 2° to 8°C (36° to 46 °F). Retain in original carton until time of use to protect from light. See package insert for dosing and dilution information.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Product of India
GUJ/DRUGS/G/28/1304
PRINCIPAL DISPLAY PANEL
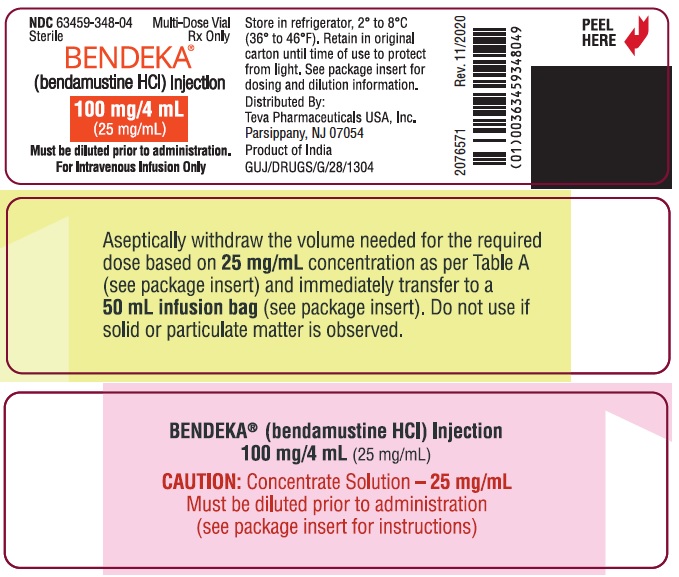
NDC 63459-348-04
Sterile
Rx Only
Multi-Dose Vial
BENDEKA™
(bendamustine HCl) Injection
100 mg/4 mL
(25 mg/mL)
For Intravenous Infusion Only
Must be diluted prior to administration.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Product of Italy
PRINCIPAL DISPLAY PANEL
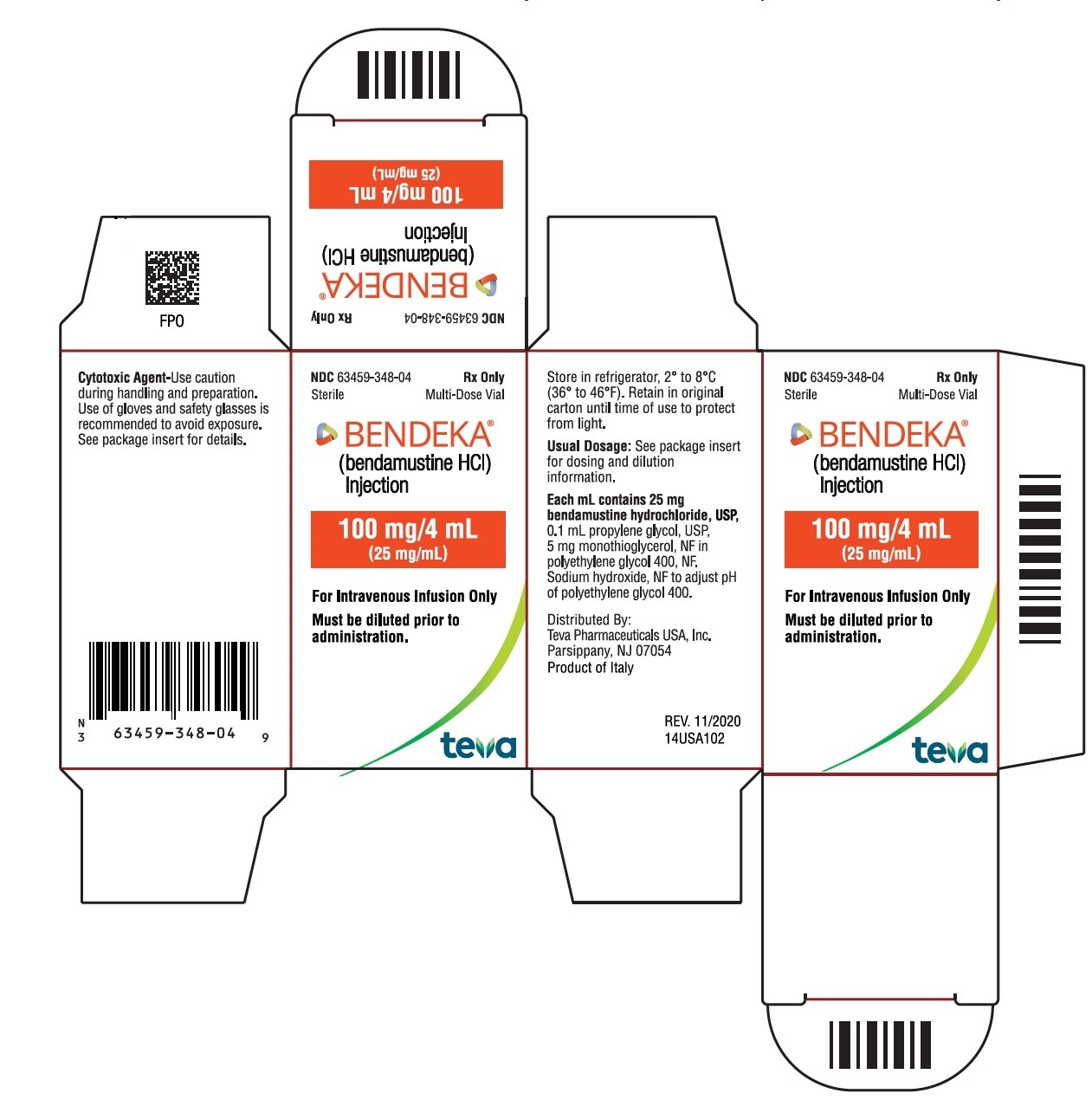
NDC 63459-348-04
Sterile
Multi-Dose Vial
Rx Only
BENDEKA™
(bendamustine HCl) Injection
100 mg/4 mL
(25 mg/mL)
Must be diluted prior to administration.
For Intravenous Infusion Only
Store in refrigerator, 2° to 8°C (36° to 46 °F). Retain in original carton until time of use to protect from light. See package insert for dosing and dilution information.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Product of Italy