NDC Code(s) : 63481-161-60, 63481-207-60, 63481-348-60, 63481-519-60, 63481-685-60, 63481-820-60, 63481-952-60
Packager : Endo Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Belbucabuprenorphine hydrochloride FILM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Belbucabuprenorphine hydrochloride FILM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Belbucabuprenorphine hydrochloride FILM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Belbucabuprenorphine hydrochloride FILM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Belbucabuprenorphine hydrochloride FILM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Belbucabuprenorphine hydrochloride FILM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Belbucabuprenorphine hydrochloride FILM | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
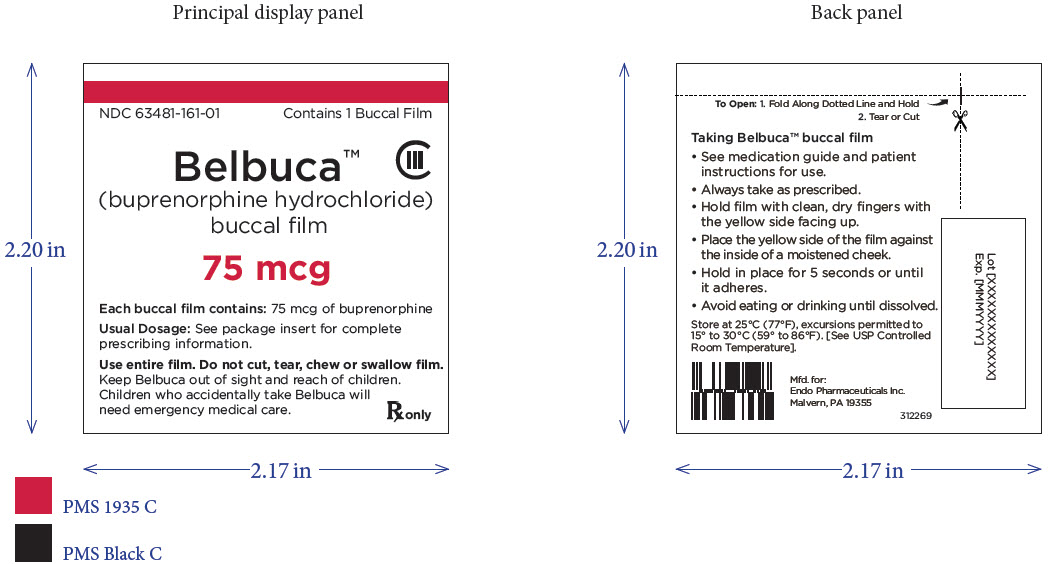
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 75ug Pouch Label
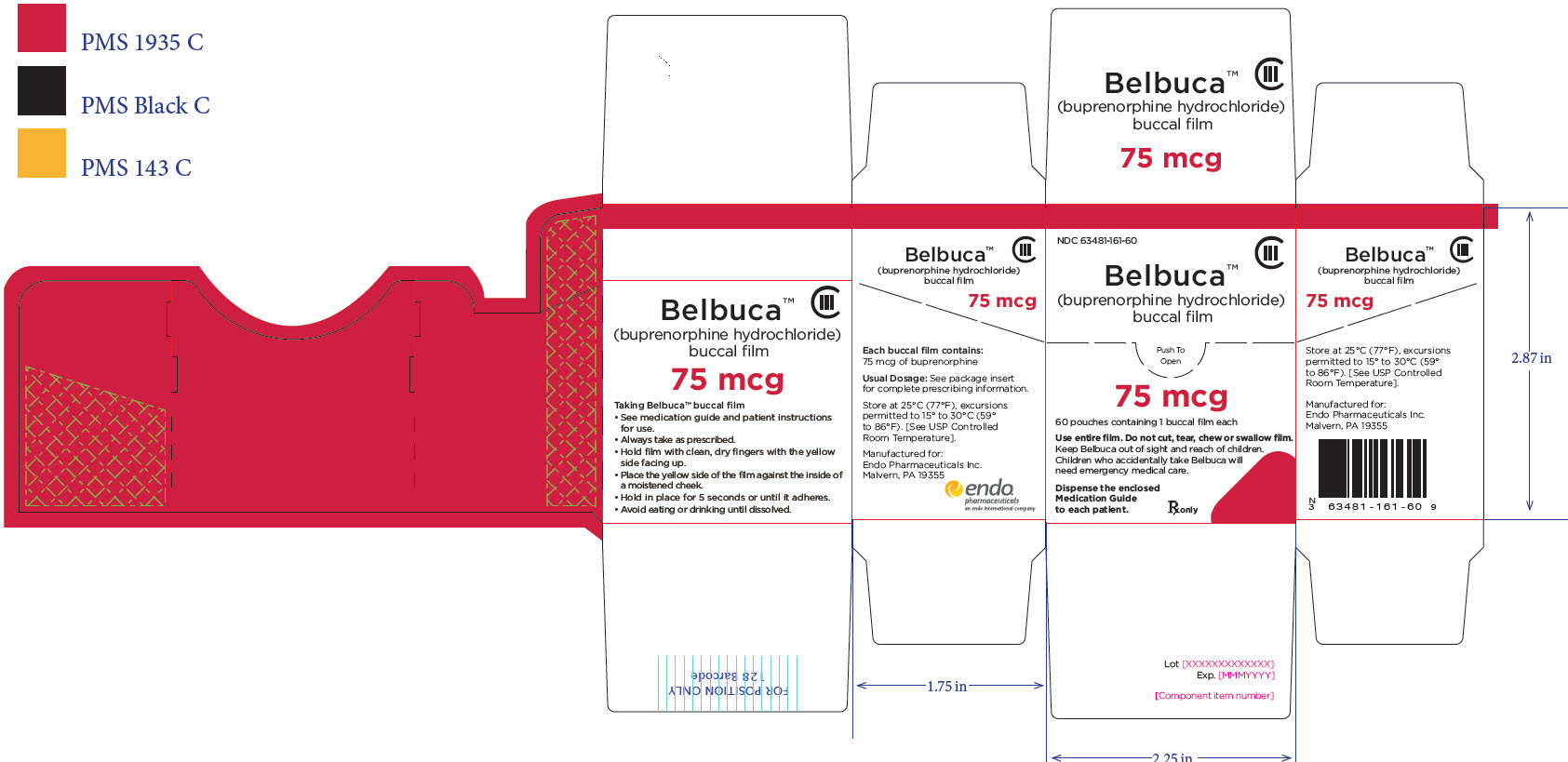
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 75ug Carton Label
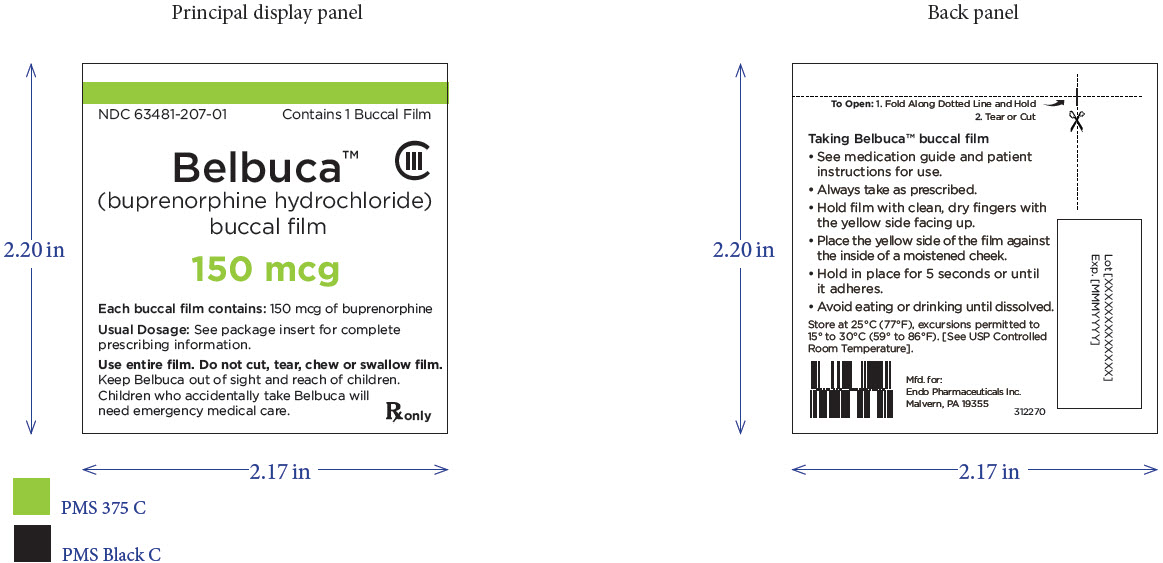
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 150ug Pouch Label
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 150ug Carton Label
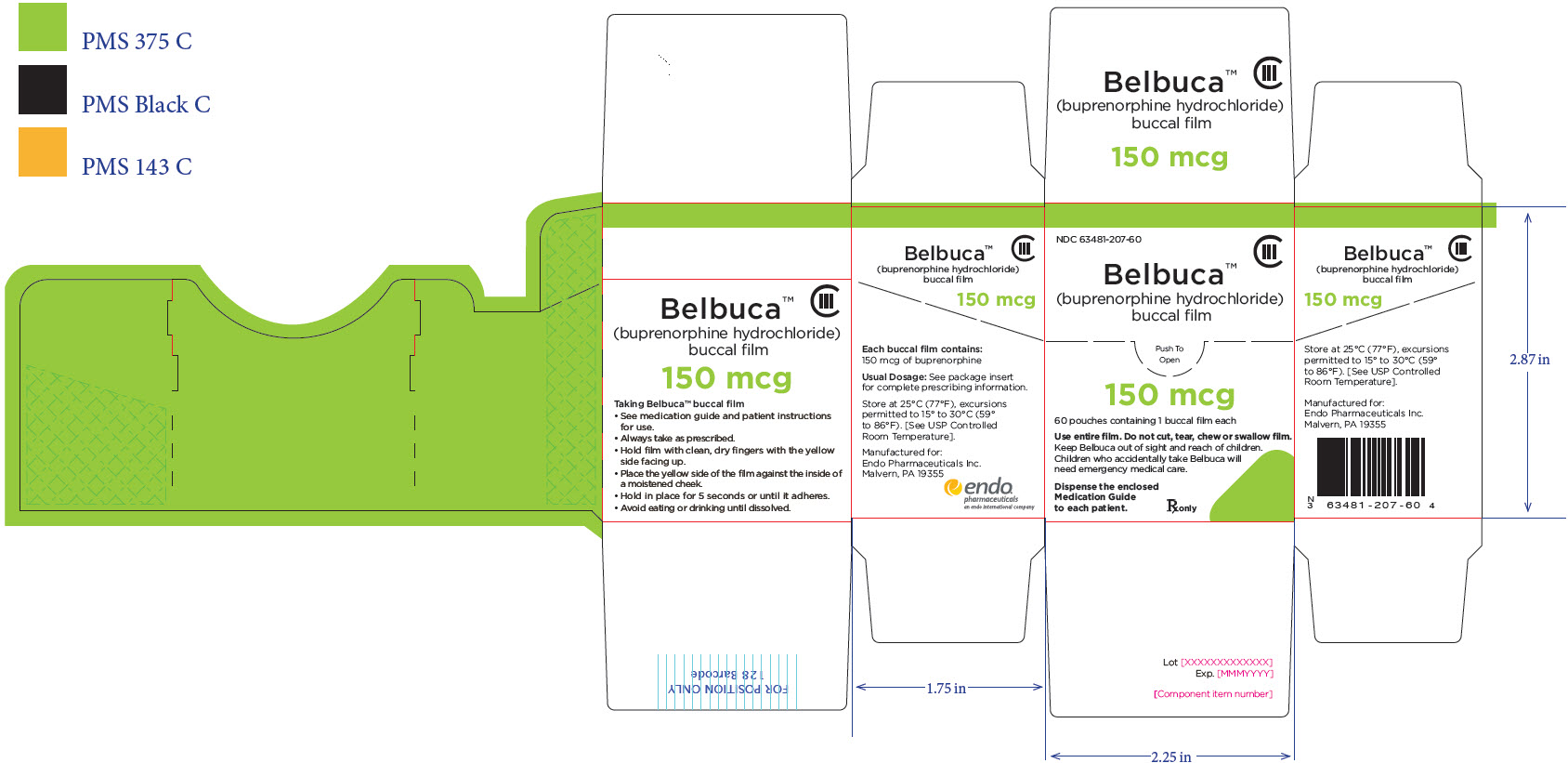
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 300ug Pouch Label
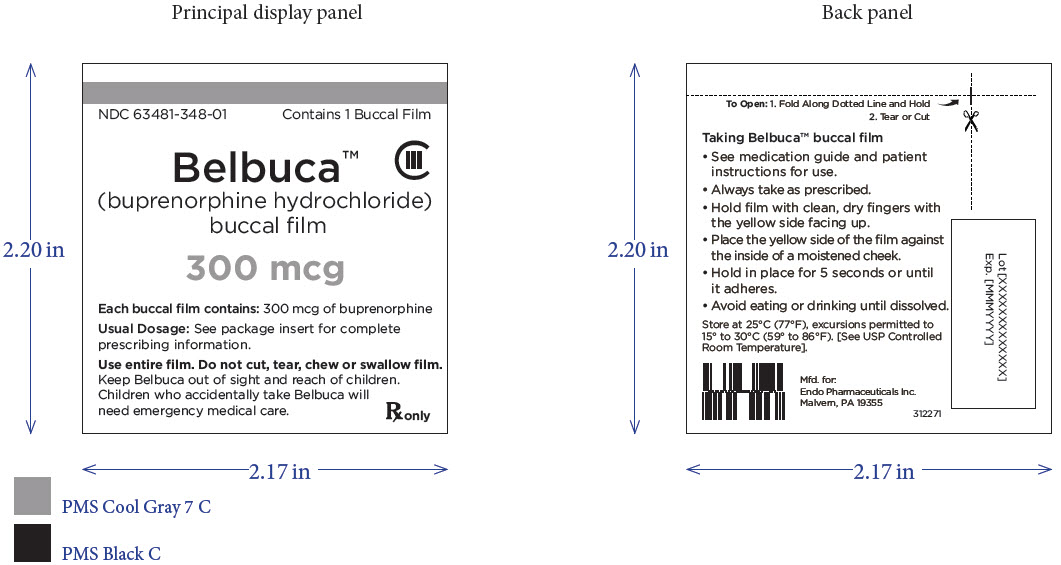
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 300ug Carton Label
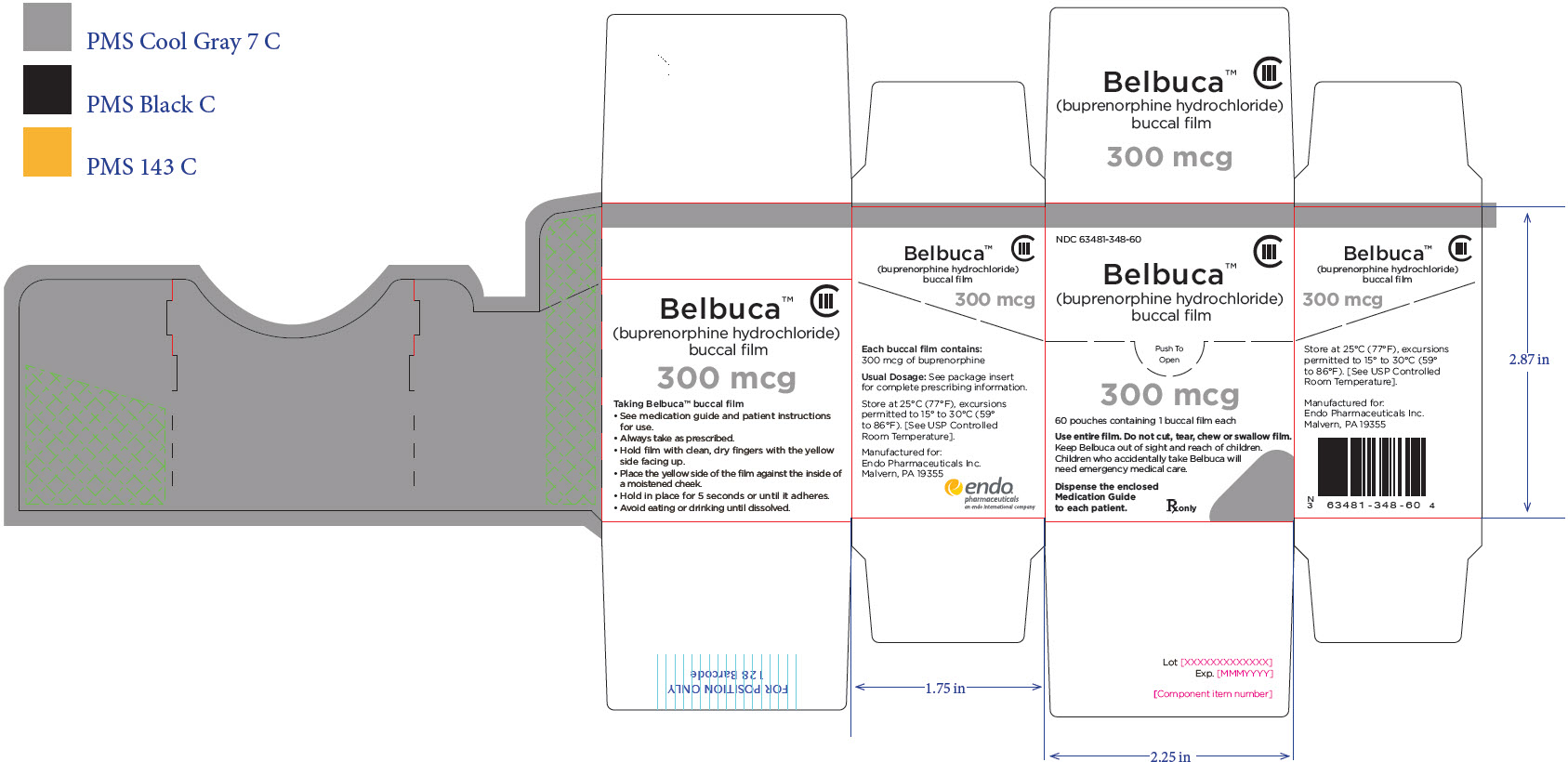
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 450ug Pouch Label
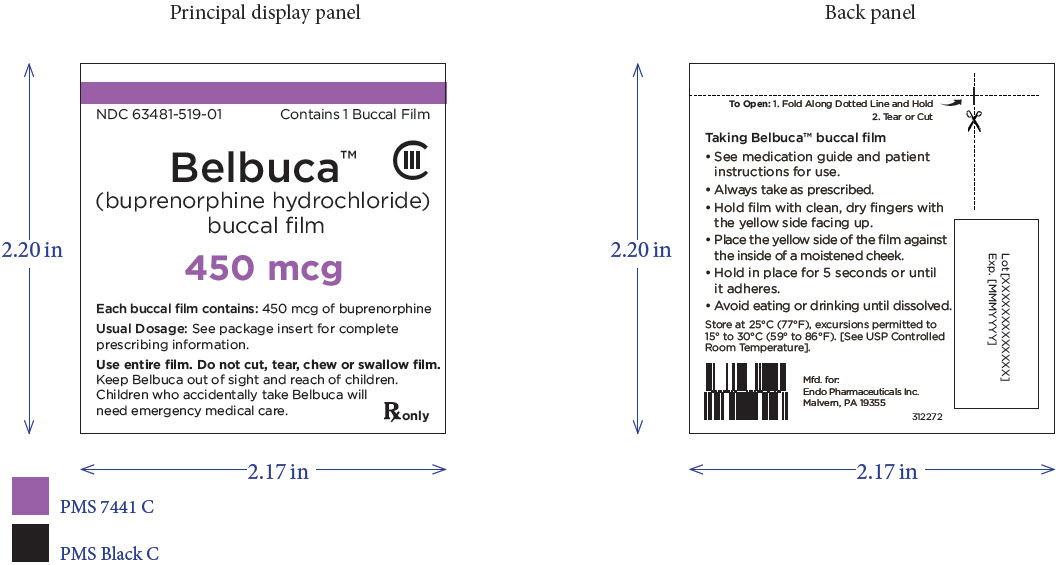
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 450ug Carton Label
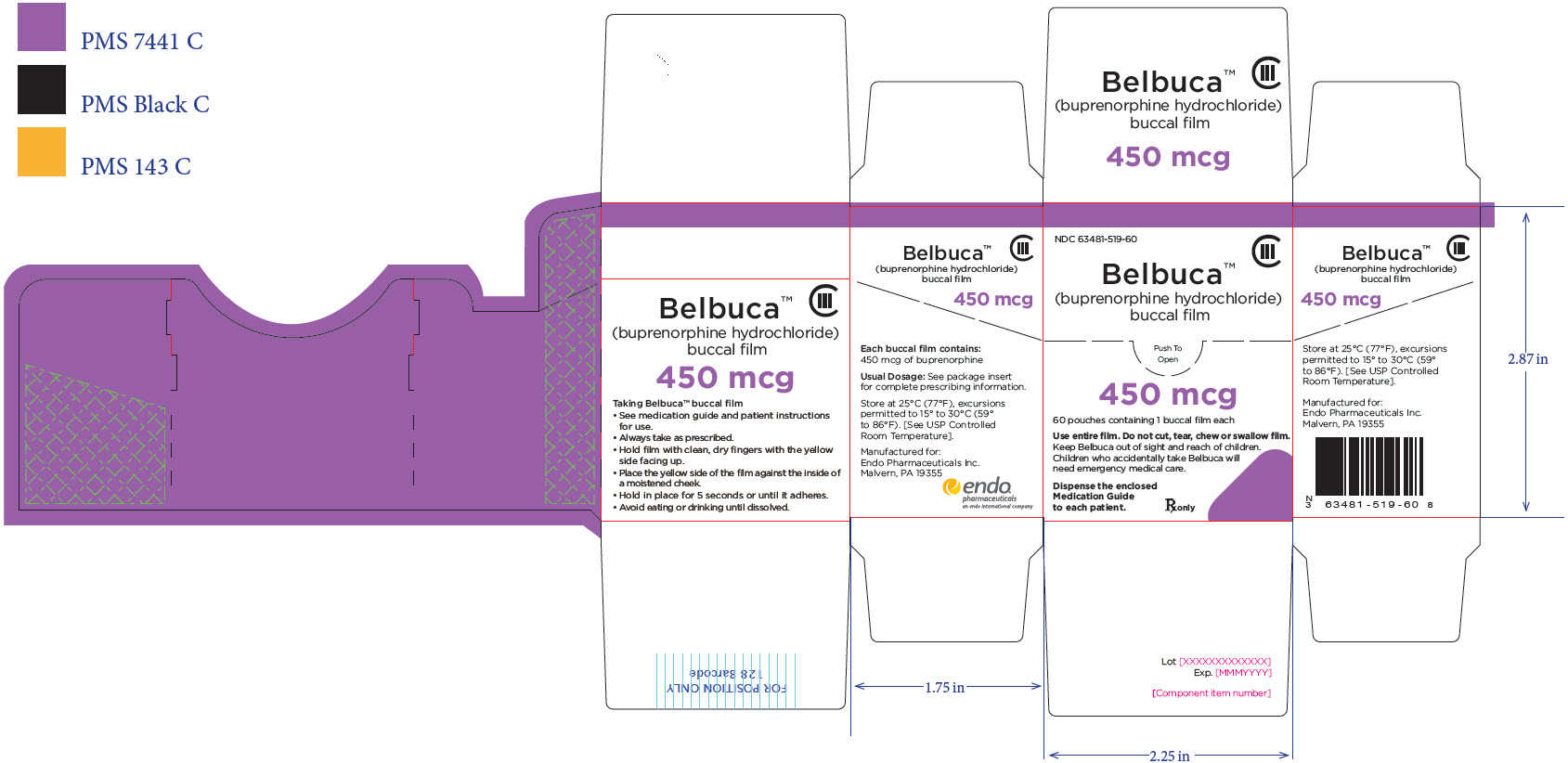
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 600ug Pouch Label
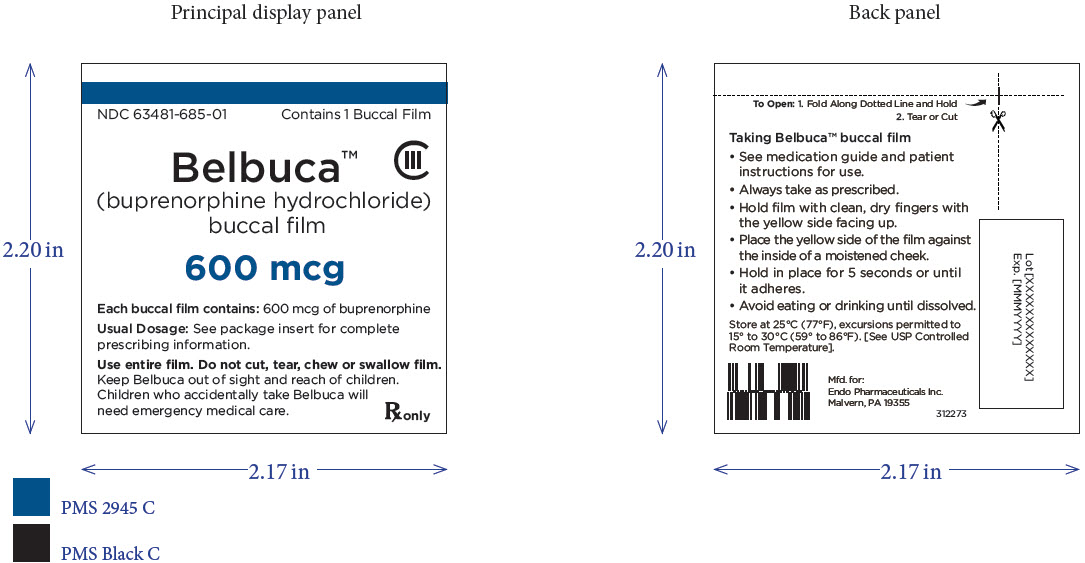
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 600ug Carton Label
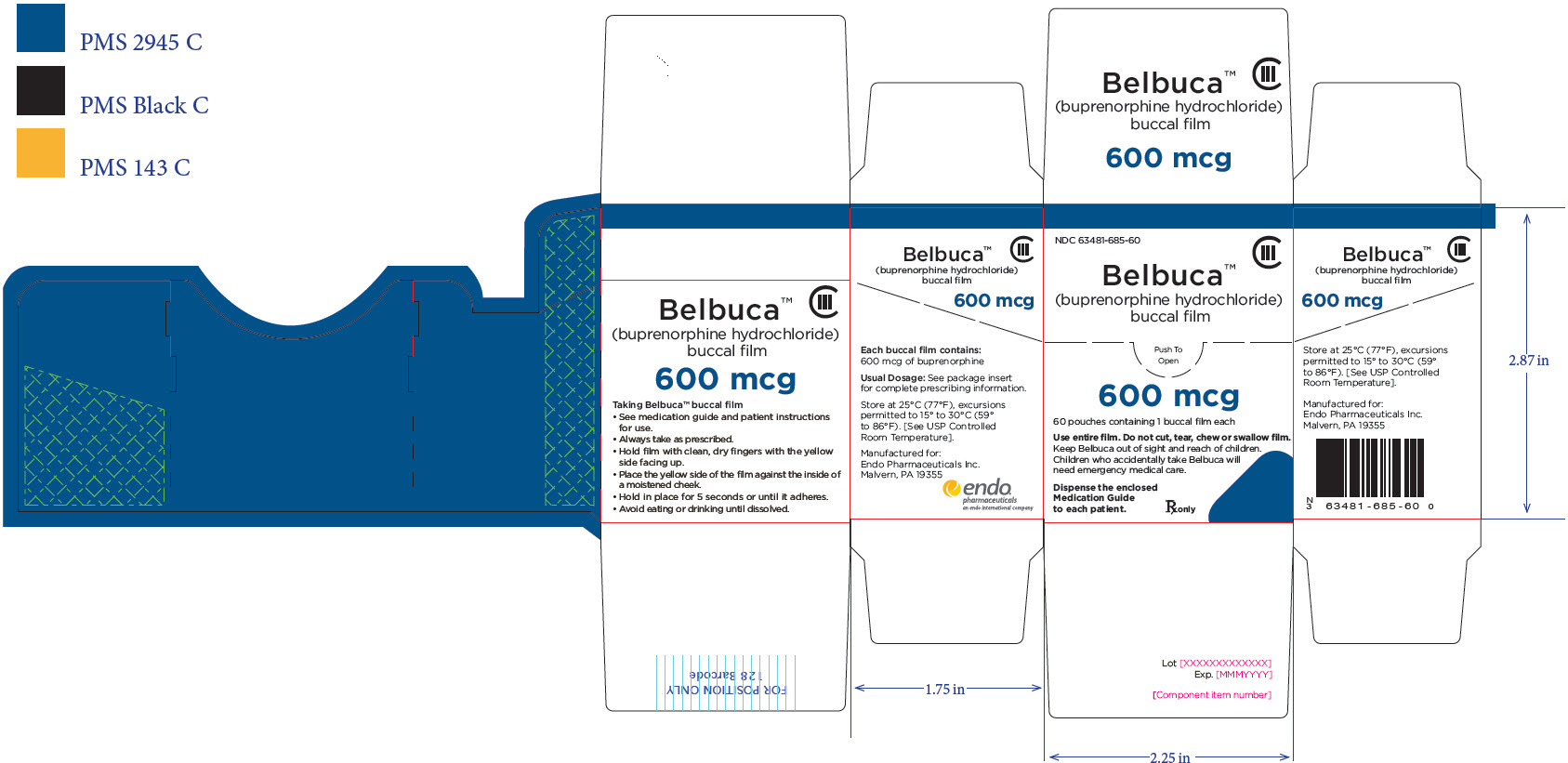
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 750ug Pouch Label
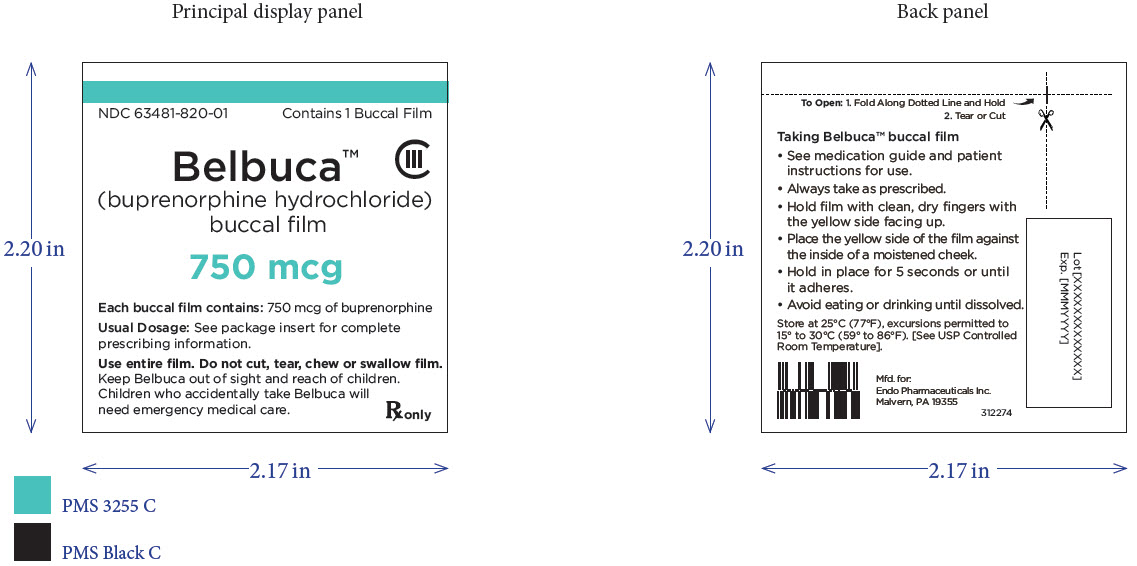
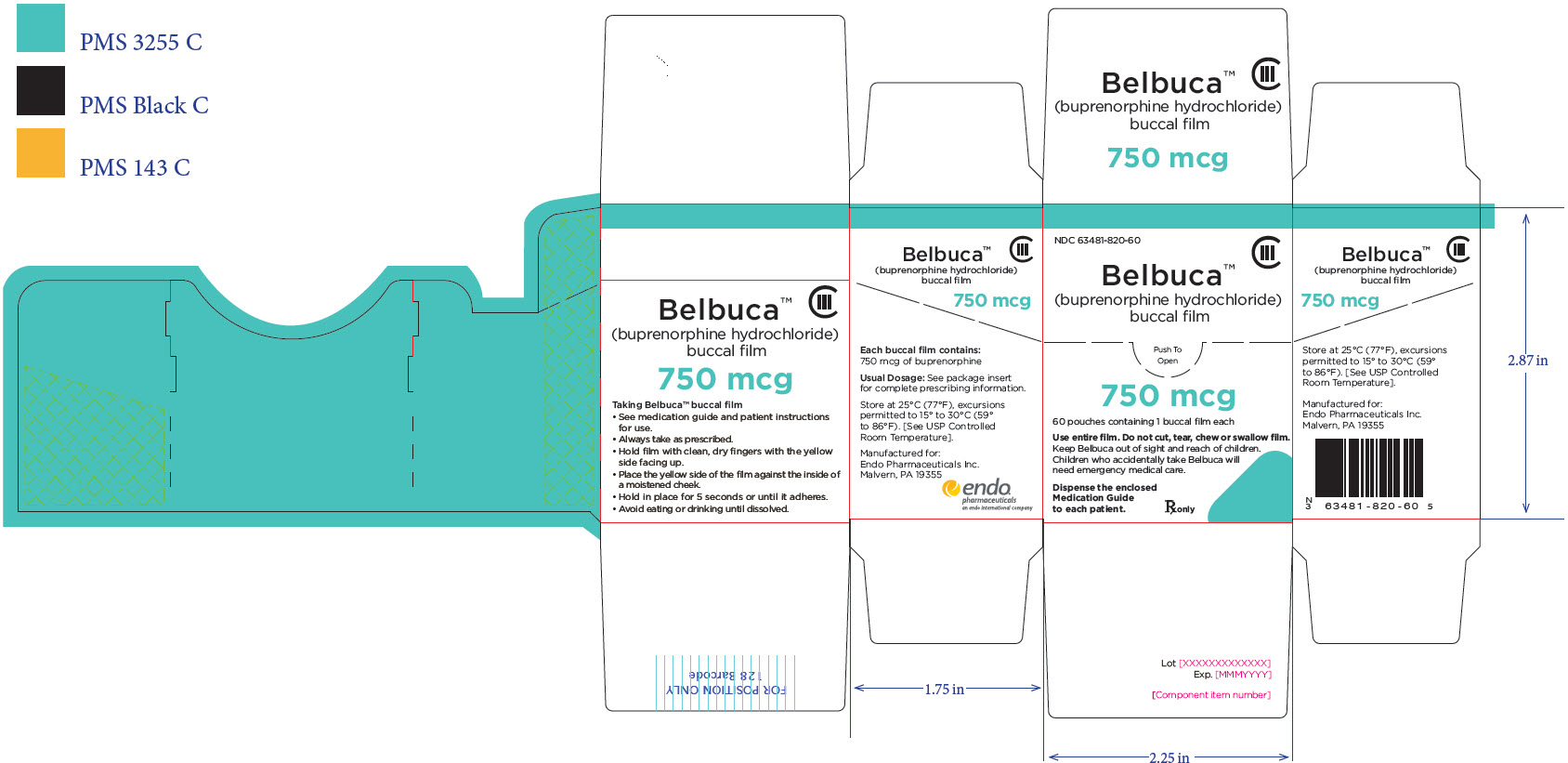
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 750ug Carton Label
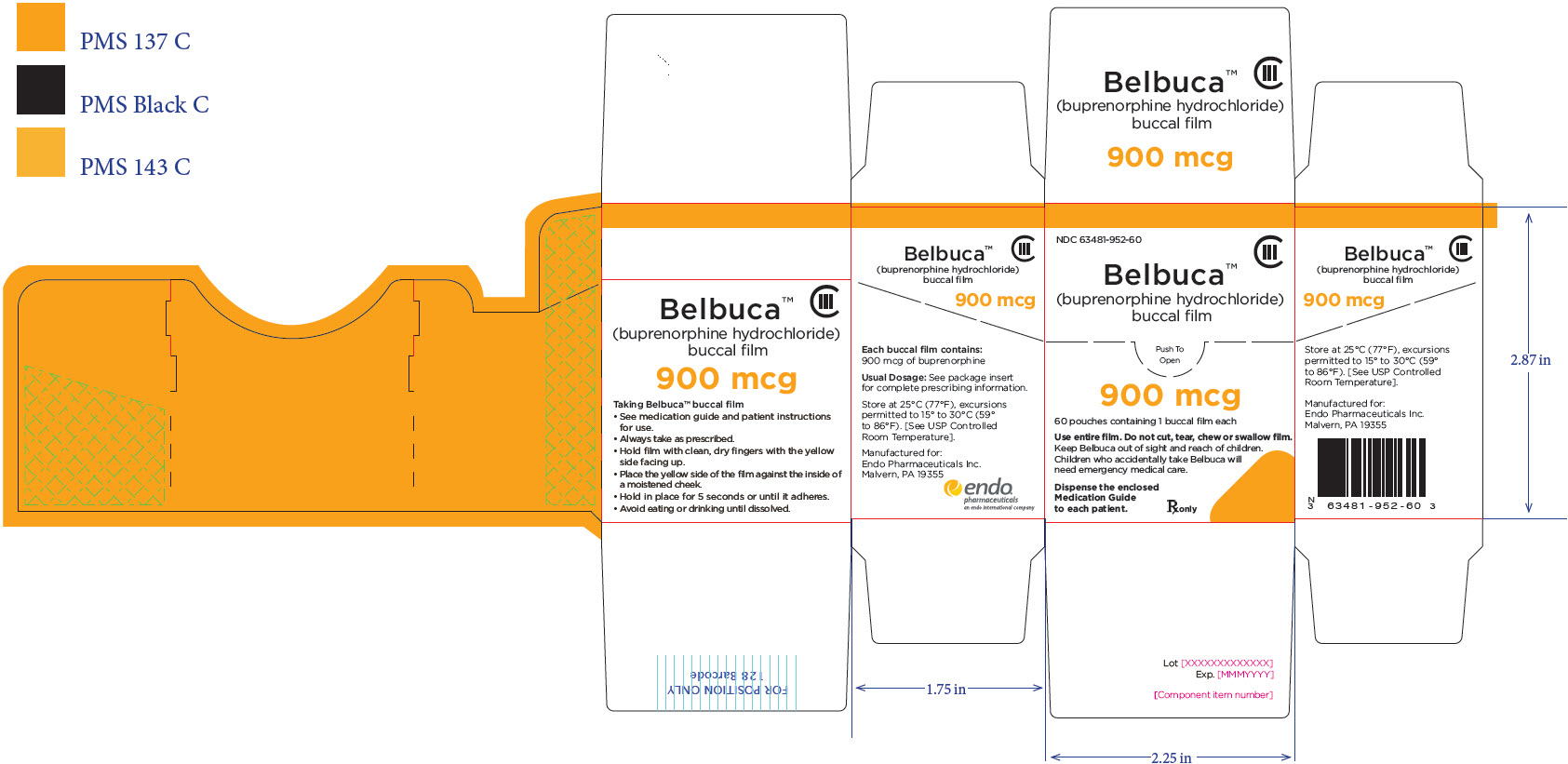
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 900ug Pouch Label
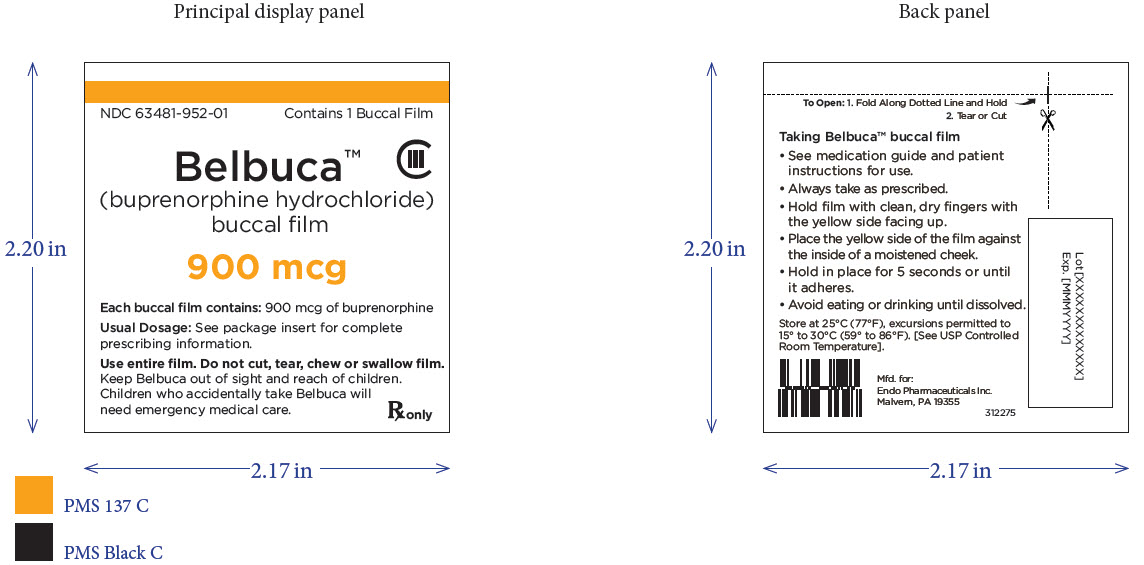
PRINCIPAL DISPLAY PANEL
Package Label – Principle Display Panel – 900ug Carton Label