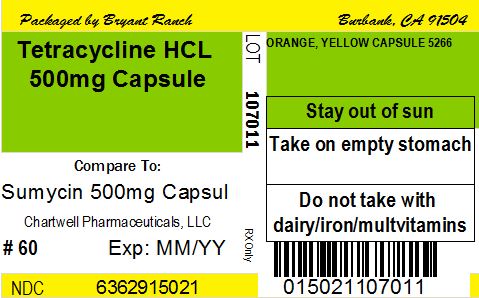
NDC Code(s) : 63629-1502-2, 63629-1502-6, 63629-1502-7, 63629-1502-5, 63629-1502-4, 63629-1502-3, 63629-1502-8, 63629-1502-1
Packager : Bryant Ranch Prepack
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Tetracycline HydrochlorideTetracycline Hydrochloride CAPSULE | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL