NDC Code(s) : 63868-940-08
Packager : Chain Drug Marketing Association Inc.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Antiseptic Skin CleanserChlorhexidine Gluconate 4% LIQUID | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Chain Drug Marketing Association Inc.(011920774) |
| REGISTRANT - Xttrium Laboratories, Inc.(007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Xttrium Laboratories, Inc. | 007470579 | manufacture(63868-940) | |
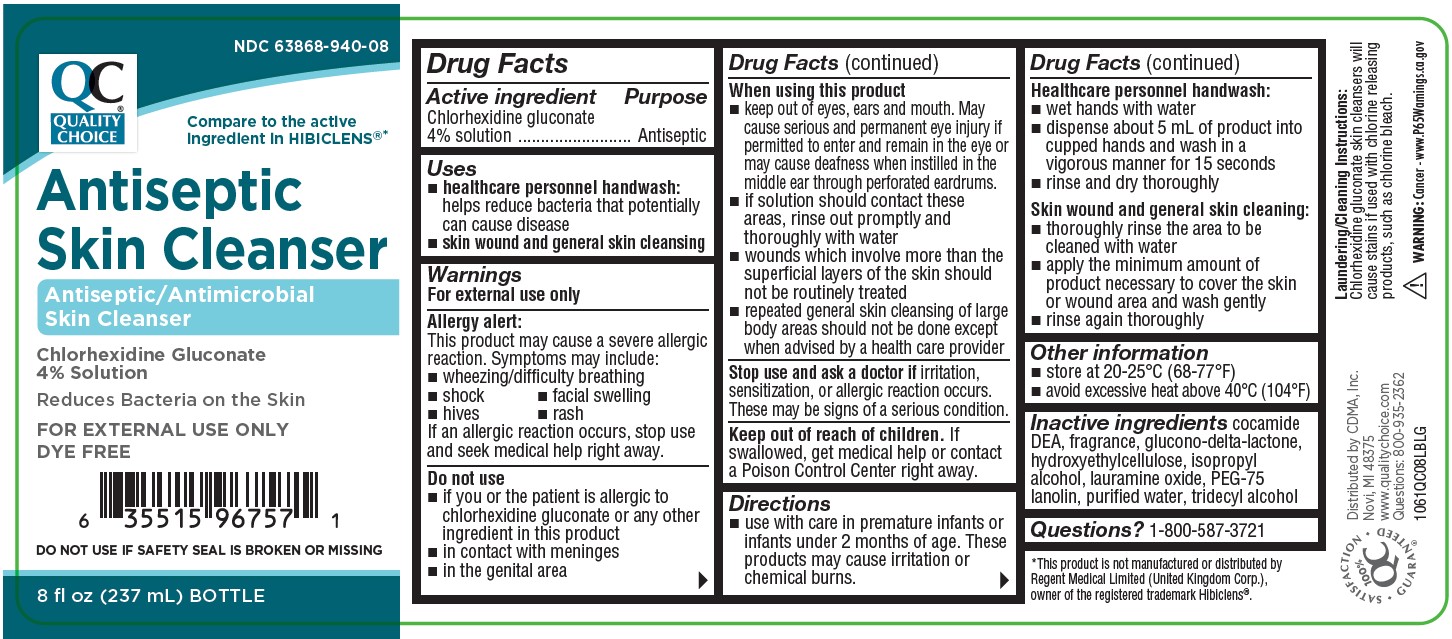
PRINCIPAL DISPLAY PANEL
Quality Choice
NDC 63868-940-08
Compare to the active ingredient in HIBICLENS®*
Antiseptic Skin Cleanser
Antiseptic/Antimicrobial Skin Cleanser
Chlorhexidine Gluconate 4% Solution
Reduces Bacteria on the Skin
Net Contents: 8FL OZ (237 mL) BOTTLE
FOR EXTERNAL USE ONLY
DYE FREE
1061QC08LBLG