NDC Code(s) : 64127-175-01
Packager : Laboratoires dermo Cosmetik Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| G.M. Collin Salicylic Acid GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
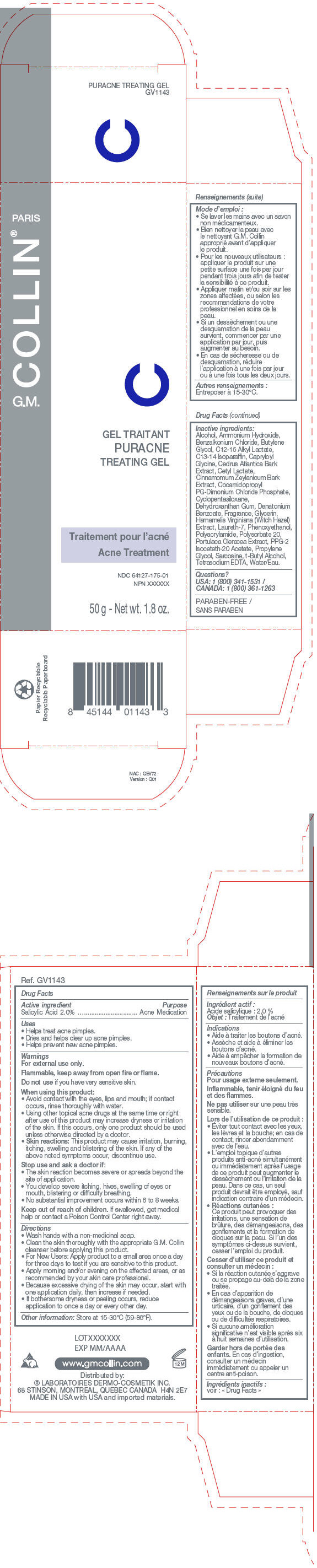
PRINCIPAL DISPLAY PANEL
PARIS
G.M. COLLIN®
PURACNE
TREATING GEL
Acne Treatment
NDC 64127-175-01
NPN XXXXXX
50 g - Net wt. 1.8 oz.