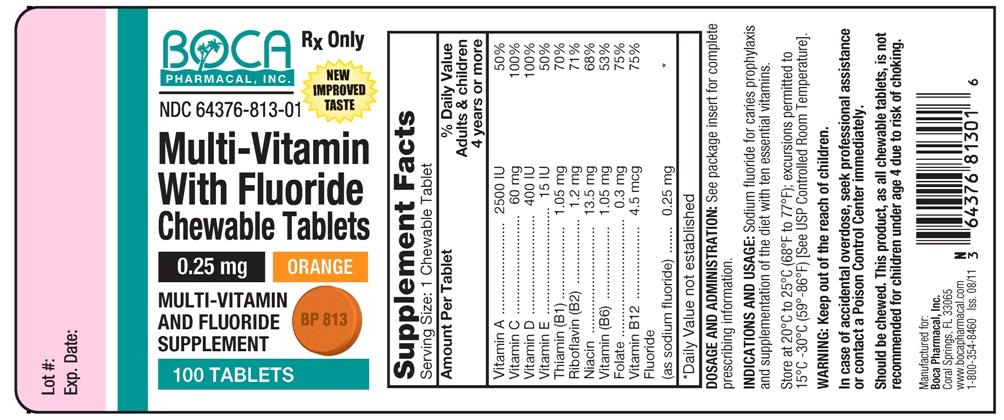
NDC Code(s) : 64376-813-01
Packager : Boca Pharmacal, Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Multi-Vitamin With Fluoride Vitamin A, Vitamin D, Thiamine, Riboflavin, Niacin, Pyridoxine, Folic Acid, Fluoride Ion, Ascorbic Acid, .Alpha.-Tocopherol-Acetate-DL, Cyanocobalamin TABLET, CHEWABLE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
[Rev 18]









