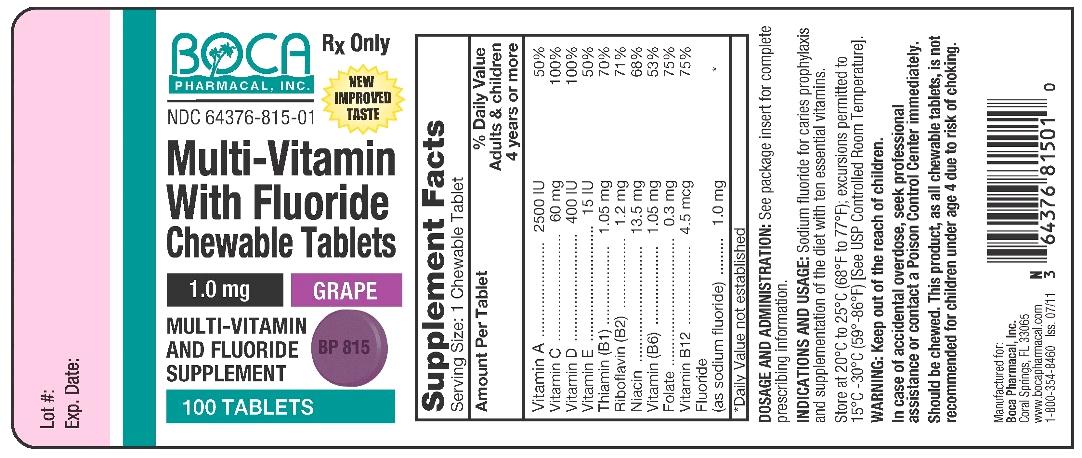
NDC Code(s) : 64376-815-01
Packager : Boca Pharmacal, Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Multi-Vitamin Vitamin A, Vitamin D, Thiamine, Riboflavin, Niacin, Pyridoxine, Folic Acid, Fluoride Ion, Ascorbic Acid, Alpha-Tocopherol-Acetate-DL, Cyanocobalamin TABLET, CHEWABLE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
[Rev 13]









