NDC Code(s) : 64380-841-06, 64380-841-11
Packager : Strides Pharma Science Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| diclofenac potassium diclofenac potassium CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Strides Pharma Science Limited(650738743) |
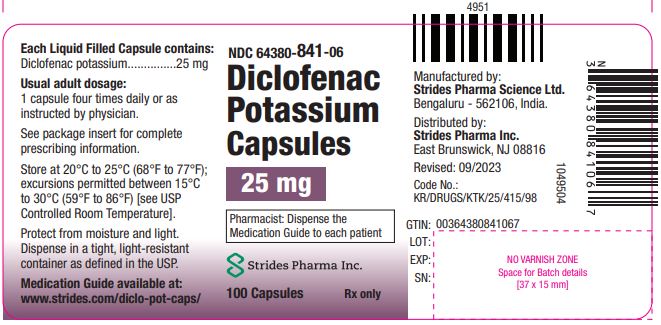
PRINCIPAL DISPLAY PANEL
NDC 64380-841-06
100 Liquid Filled Capsules
Rx Only
Diclofenac Potassium Capsules
25 mg
Marketed by: Strides Pharma Inc.
NDC 64380-841-11
120 Liquid Filled Capsules
Rx Only
Diclofenac Potassium Capsules
25 mg
Marketed by: Strides Pharma Inc.










