NDC Code(s) : 65580-301-03, 65580-301-09, 65580-302-03, 65580-302-09, 65580-303-03, 65580-303-09, 65580-304-03, 65580-304-09
Packager : Upstate Pharma, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| venlafaxine hydrochloridevenlafaxine hydrochloride TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| venlafaxine hydrochloridevenlafaxine hydrochloride TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| venlafaxine hydrochloridevenlafaxine hydrochloride TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| venlafaxine hydrochloridevenlafaxine hydrochloride TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
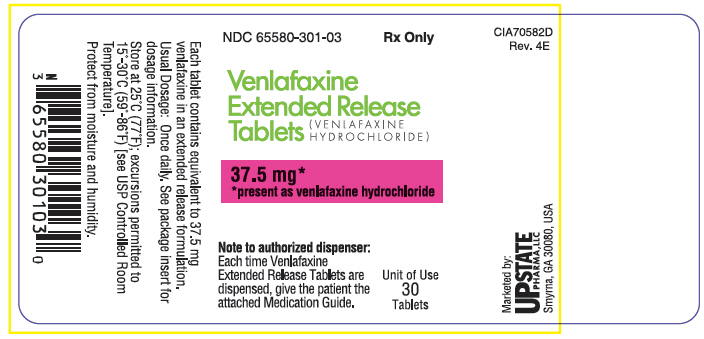
PRINCIPAL DISPLAY PANEL
NDC 65580-301-03
Rx Only
Venlafaxine
Extended Release
Tablets
(VENLAFAXINE
HYDROCHLORIDE)
37.5 mg*
*present as venlafaxine hydrochloride
Note to authorized dispenser:
Each time Venlafaxine
Extended Release Tablets are
dispensed, give the patient the
attached Medication Guide.
Unit of Use
30
Tablets
PRINCIPAL DISPLAY PANEL
NDC 65580-302-03
Rx Only
Venlafaxine
Extended Release
Tablets
(VENLAFAXINE
HYDROCHLORIDE)
75 mg*
*present as venlafaxine hydrochloride
Note to authorized dispenser:
Each time Venlafaxine
Extended Release Tablets are
dispensed, give the patient the
attached Medication Guide.
Unit of Use
30
Tablets

PRINCIPAL DISPLAY PANEL
NDC 65580-303-03
Rx Only
Venlafaxine
Extended Release
Tablets
(VENLAFAXINE
HYDROCHLORIDE)
150 mg*
*present as venlafaxine hydrochloride
Note to authorized dispenser:
Each time Venlafaxine
Extended Release Tablets are
dispensed, give the patient the
attached Medication Guide.
Unit of Use
30
Tablets

PRINCIPAL DISPLAY PANEL
NDC 65580-304-03
Rx Only
Venlafaxine
Extended Release
Tablets
(VENLAFAXINE
HYDROCHLORIDE)
225 mg*
*present as venlafaxine hydrochloride
Note to authorized dispenser:
Each time Venlafaxine
Extended Release Tablets are
dispensed, give the patient the
attached Medication Guide.
Unit of Use
30
Tablets










