NDC Code(s) : 65862-218-60, 65862-218-01, 65862-219-60, 65862-219-01
Packager : Aurobindo Pharma Limited
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cefdinir Cefdinir POWDER, FOR SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Cefdinir Cefdinir POWDER, FOR SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Aurobindo Pharma Limited(650082092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aurobindo Pharma Limited | 918917639 | ANALYSIS(65862-218, 65862-219), MANUFACTURE(65862-218, 65862-219) | |
PRINCIPAL DISPLAY PANEL
NDC 65862-218-60
Rx only
Cefdinir for Oral
Suspension, USP
125 mg/5 mL
SHAKE WELL BEFORE USING.
Keep bottle tightly closed.
Any unused portion must bediscarded
10 days after mixing.
RECONSTITUTE WITH 38 mL WATER
60 mL when reconstituted
AUROBINDO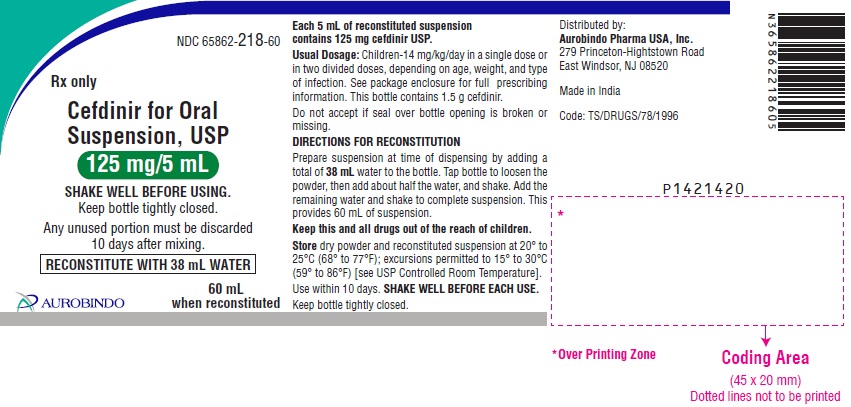
PRINCIPAL DISPLAY PANEL
Rx only NDC 65862-218-60
Cefdinir for Oral
Suspension, USP
125 mg/5 mL
SHAKE WELL BEFORE USING.
Keep bottle tightly closed.
Any unused portion must be
discarded 10 days after mixing.
RECONSTITUTE WITH
38 mL WATER
60 mL when reconstituted
AUROBINDO

PRINCIPAL DISPLAY PANEL
NDC 65862-219-60
Rx only
Cefdinir for Oral
Suspension, USP
250 mg/5 mL
SHAKE WELL BEFORE USING.
Keep bottle tightly closed.
Any unused portion must be discarded
10 days after mixing.
RECONSTITUTE WITH 38 mL WATER
60 mL
when reconstituted
AUROBINDO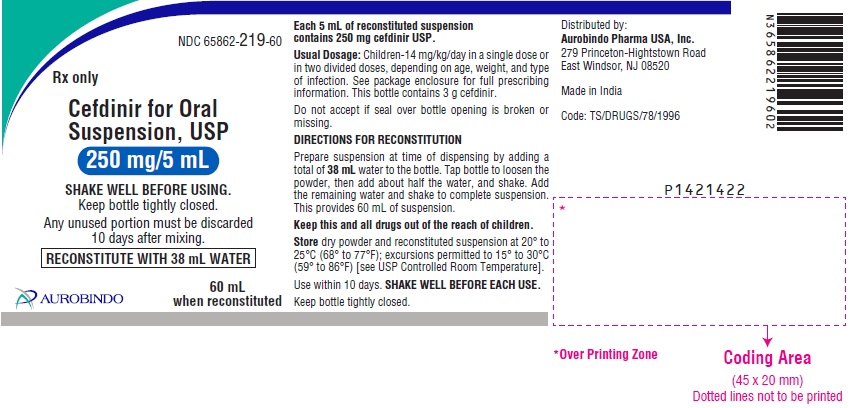
PRINCIPAL DISPLAY PANEL
Rx only NDC 65862-219-60
Cefdinir for Oral
Suspension, USP
250 mg/5 mL
SHAKE WELL BEFORE USING.
Keep bottle tightly closed.
Any unused portion must be
discarded 10 days after mixing.
RECONSTITUTE WITH
38 mL WATER
60 mL
when reconstituted
AUROBINDO










