NDC Code(s) : 65862-934-87, 65862-934-88, 65862-934-58
Packager : Aurobindo Pharma Limited
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Aurovela 24 FeNorethindrone Acetate and Ethinyl Estradiol and Ferrous Fumarate KIT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Aurobindo Pharma Limited(650082092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(65862-934), MANUFACTURE(65862-934) | |
PRINCIPAL DISPLAY PANEL
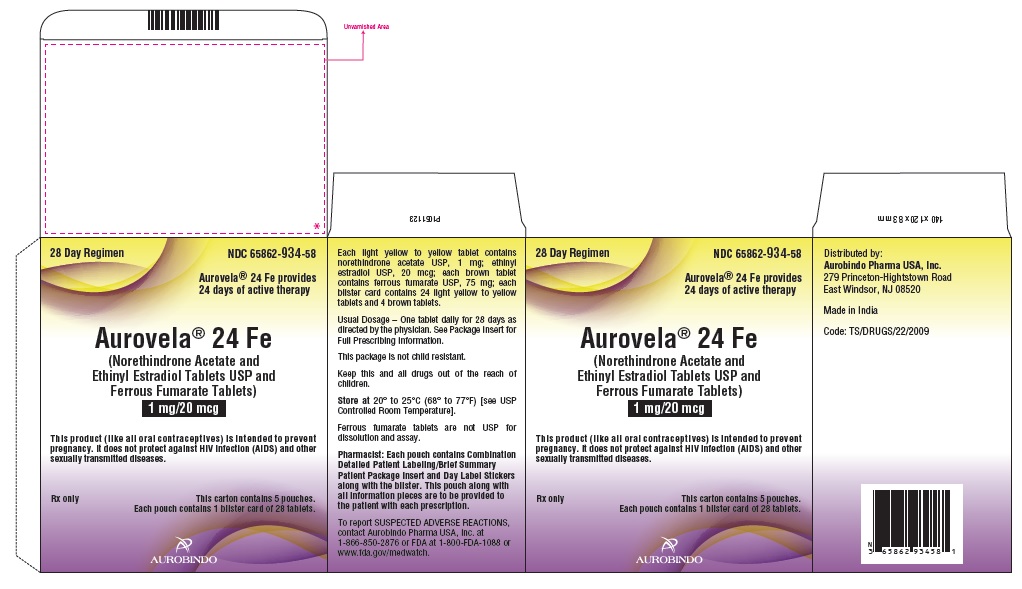
NDC 65862-934-87
28 Day Regimen
Aurovela® 24 Fe provides
24 days of active therapy
Aurovela® 24 Fe
(Norethindrone Acetate and Ethinyl Estradiol
Tablets USP and Ferrous Fumarate Tablets)
1 mg/20 mcg
Each light yellow to yellow tablet contains norethindrone
acetate USP, 1 mg: ethinyl estradiol USP, 20 mcg; each brown
tablet contains ferrous fumarate USP, 75 mg.
Each pouch contains 24 light yellow to yellow tablets and 4
brown tablets.
This product (like all oral contraceptives) is
intended to prevent pregnancy. It does not
protect against HIV infection (AIDS) and other
sexually transmitted diseases.
Rx only This pouch contains
one blister card of 28 tablets
AUROBINDO
PRINCIPAL DISPLAY PANEL
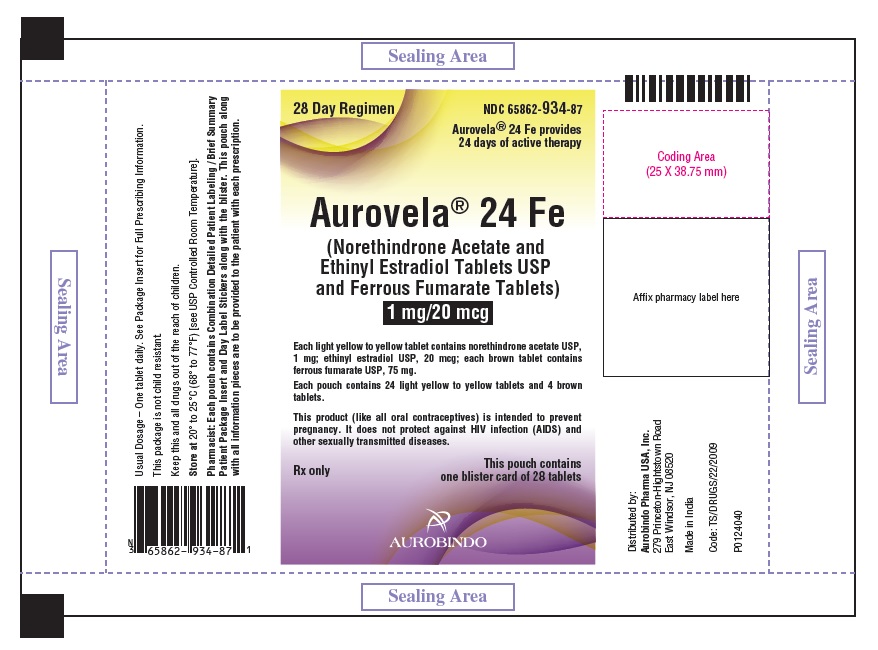
NDC 65862-934-58
28 Day Regimen
Aurovela® 24 Fe provides
24 days of active therapy
Aurovela® 24 Fe
(Norethindrone Acetate and Ethinyl Estradiol
Tablets USP
and
Ferrous Fumarate Tablets
)
1 mg/20 mcg
This product (like all oral contraceptives) is intended
to prevent pregnancy. It does not
protect against HIV
infection (AIDS) and other sexually transmitted diseases.
This carton contains 5 pouches.
Rx only Each pouch contains 1 blister card of 28 tablets.
AUROBINDO