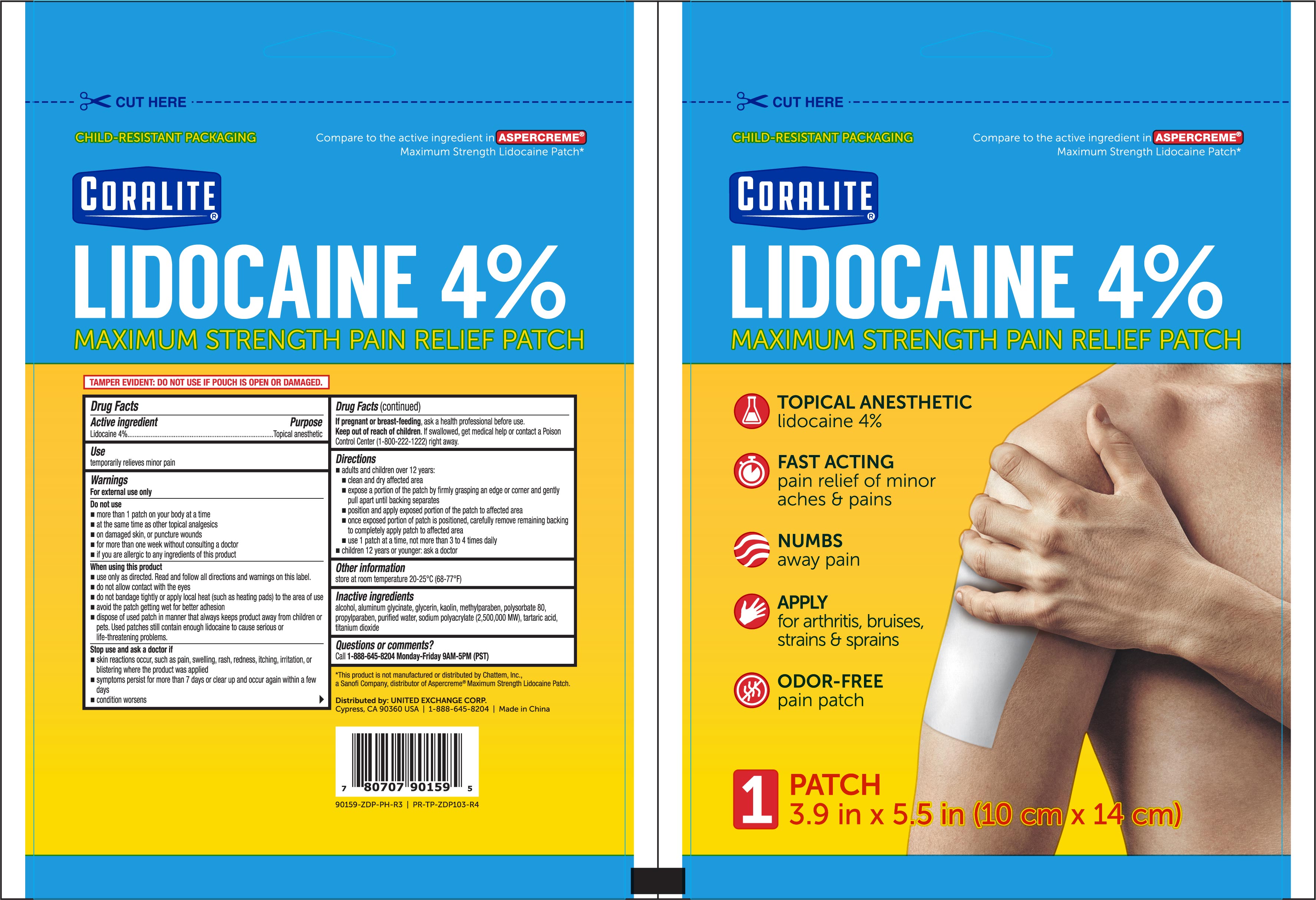
NDC Code(s) : 65923-149-01
Packager : United Exchange Corporation
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Coralite Odor Free Pain ReliefLidocaine PATCH | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - United Exchange Corporation(840130579) |
PRINCIPAL DISPLAY PANEL