NDC Code(s) : 66215-401-01
Packager : Actelion Pharmaceuticals US, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Veletriepoprostenol sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
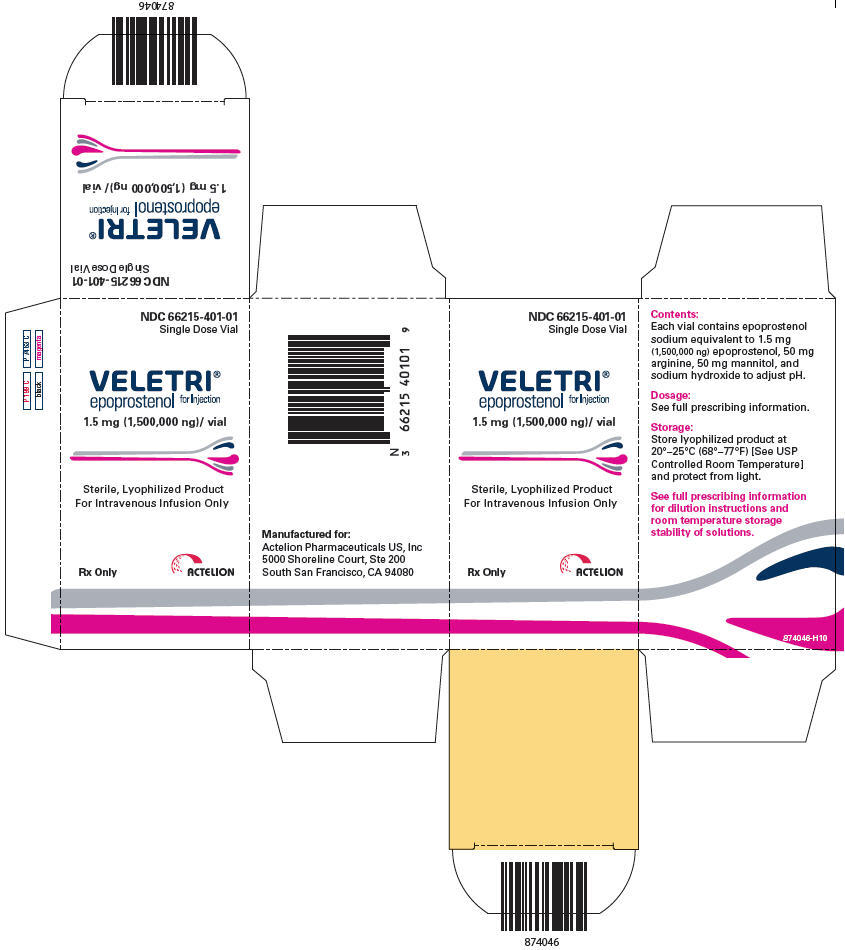
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 1.5 mg Vial Carton
NDC 66215-401-01
Single Dose Vial
VELETRI®
epoprostenol for Injection
1.5 mg (1,500,000 ng)/ vial
Sterile, Lyophilized Product
For Intravenous Infusion Only
Rx Only
ACTELION