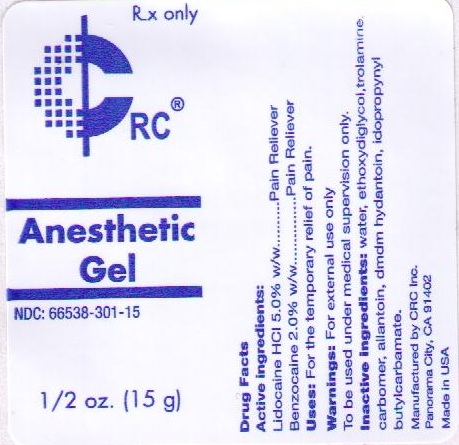
NDC Code(s) : 66538-301-15
Packager : COSMOCEUTICAL RESEARCH CENTER INC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ANESTHETIC LIDOCAINE HYDROCHLORIDE BENZOCAINE GEL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
RX ONLY
CRC
ANESTHETIC GEL
NDC: 66538-301-15
1/2 OZ. (15 ML)