NDC Code(s) : 66658-501-01, 66658-505-01, 66658-510-01, 66658-522-01, 66658-523-01, 66658-524-01, 66658-525-01
Packager : Swedish Orphan Biovitrum AB (publ)
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| GAMIFANTemapalumab-lzsg INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GAMIFANTemapalumab-lzsg INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GAMIFANTemapalumab-lzsg INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GAMIFANTemapalumab-lzsg INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GAMIFANTemapalumab-lzsg INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GAMIFANTemapalumab-lzsg INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GAMIFANTemapalumab-lzsg INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Swedish Orphan Biovitrum AB (publ)(354010589) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Patheon Italia S.p.A | 434078638 | MANUFACTURE(66658-501, 66658-505, 66658-510, 66658-522, 66658-523, 66658-524, 66658-525) | |
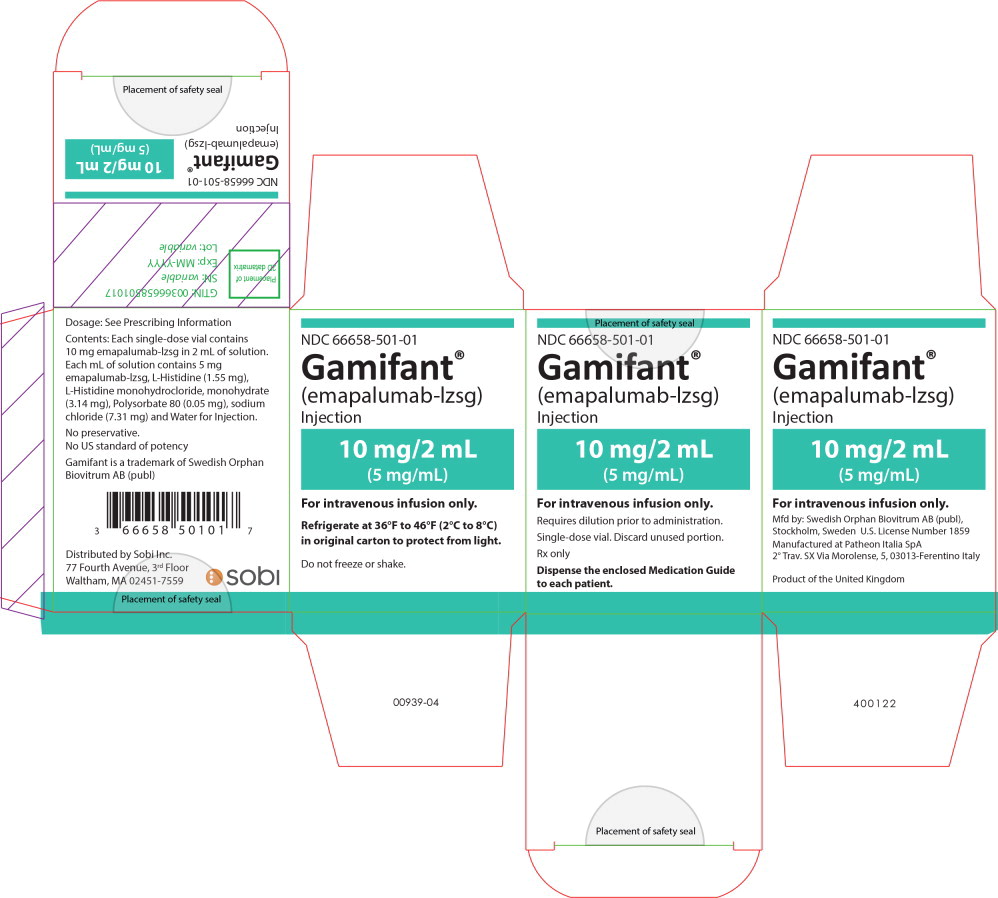
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 10mg/2mL Carton Label
NDC 66658-501-01
Gamifant
®
(emapalumab-lzsg)
Injection
10 mg/2 mL
(5 mg/mL)
For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient.
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 50mg/10mL Carton Label
NDC 66658-505-01
Gamifant
®
(emapalumab-lzsg)
Injection
50 mg/10 mL
(5 mg/mL)
For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient.

PRINCIPAL DISPLAY PANEL
Principal Display Panel - 100mg/20mL Carton Label
NDC 66658-510-01
Gamifant
®
(emapalumab-lzsg)
Injection
100 mg/20 mL
(5 mg/mL)
For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient.

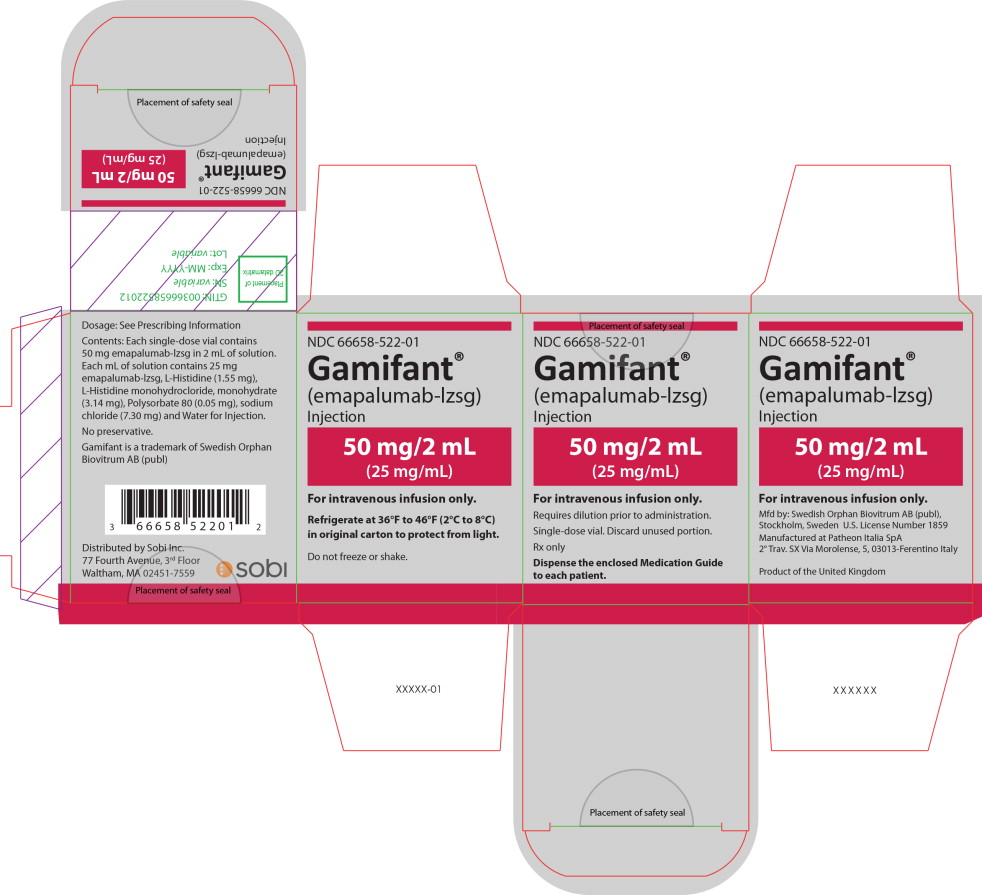
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 50 mg/2 mL Carton Label
NDC 66658-522-01
Gamifant ®
(emapalumab-lzsg)
Injection
50 mg/2 mL
(25 mg/mL)
For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient.
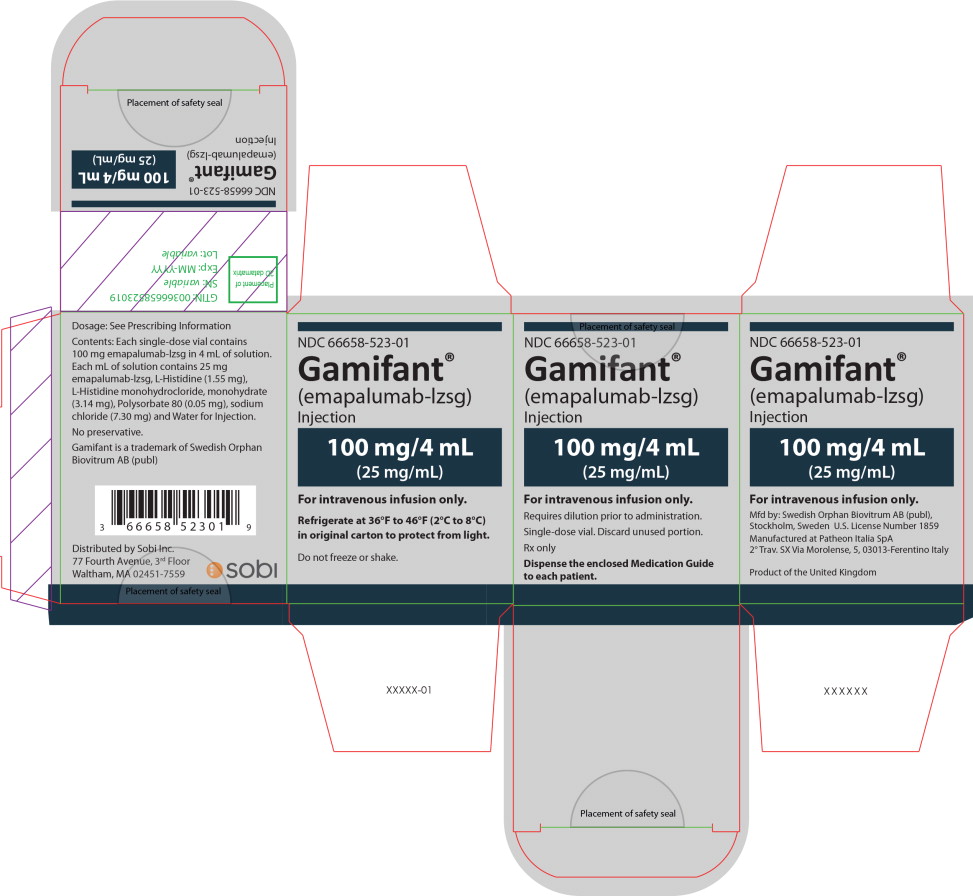
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 100 mg/4 mL Carton Label
NDC 66658-523-01
Gamifant ®
(emapalumab-lzsg)
Injection
100 mg/4 mL
(25 mg/mL)
For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient.
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 250 mg/10 mL Carton Label
NDC 66658-524-01
Gamifant ®
(emapalumab-lzsg)
Injection
250 mg/10 mL
(25 mg/mL)
For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient.

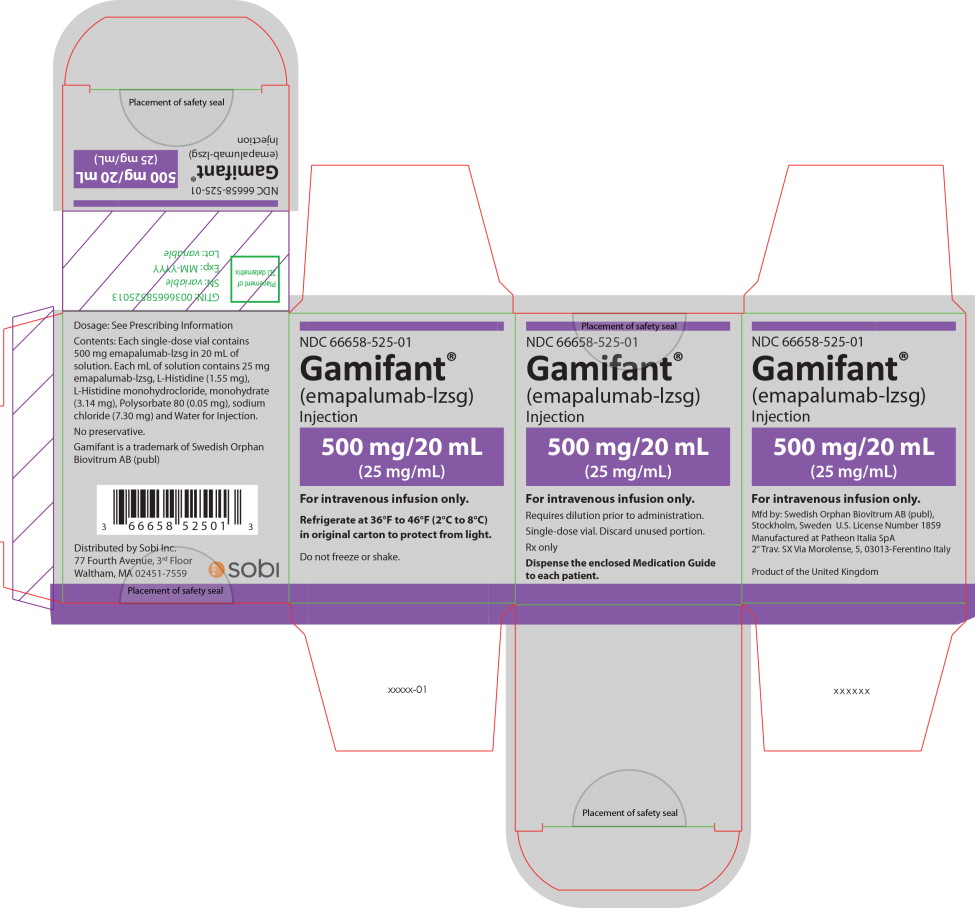
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 500 mg/20 mL Carton Label
NDC 66658-525-01
Gamifant ®
(emapalumab-lzsg)
Injection
500 mg/20 mL
(25 mg/mL)
For intravenous infusion only.
Requires dilution prior to administration.
Single-dose vial. Discard unused portion.
Rx only
Dispense the enclosed Medication Guide
to each patient.