NDC Code(s) : 66689-735-05
Packager : VistaPharm, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AripiprazoleAripiprazole SOLUTION | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - VistaPharm, Inc.(116743084) |
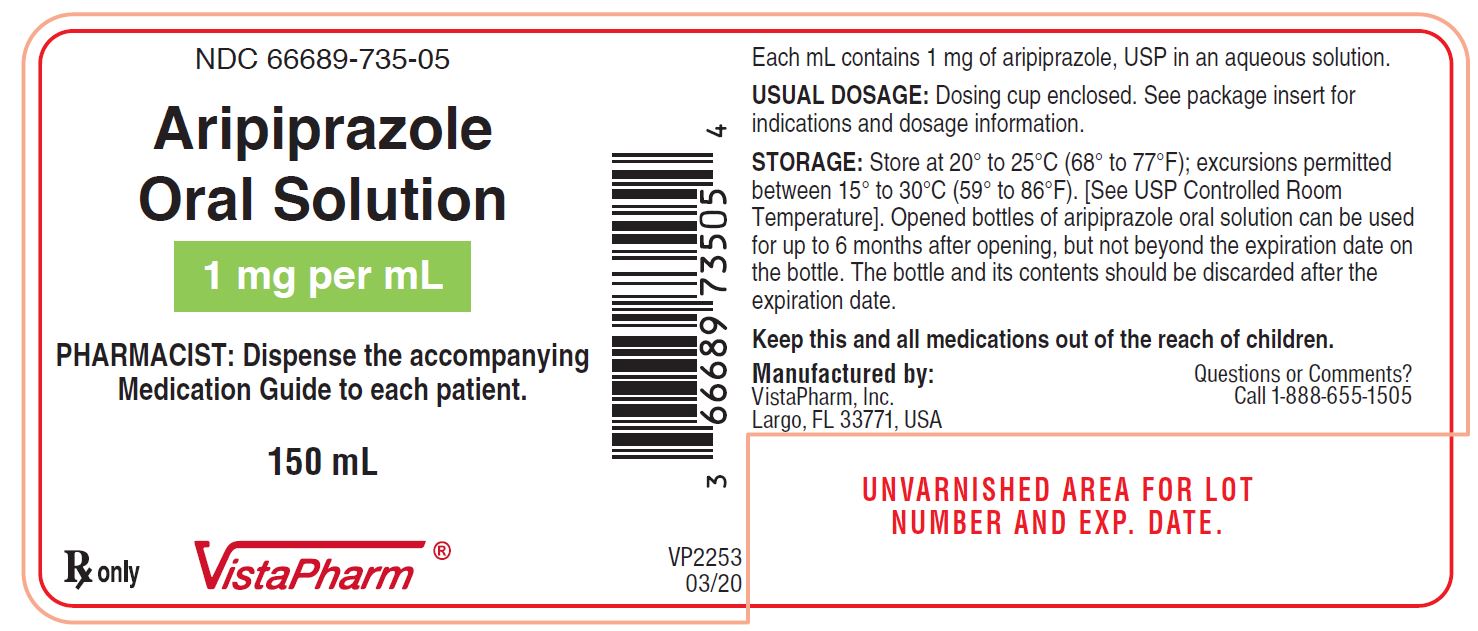
PRINCIPAL DISPLAY PANEL
NDC 66689-735-05
Aripiprazole
Oral Solution
1 mg per mL
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
150 mL
Rx Only
VistaPharm®
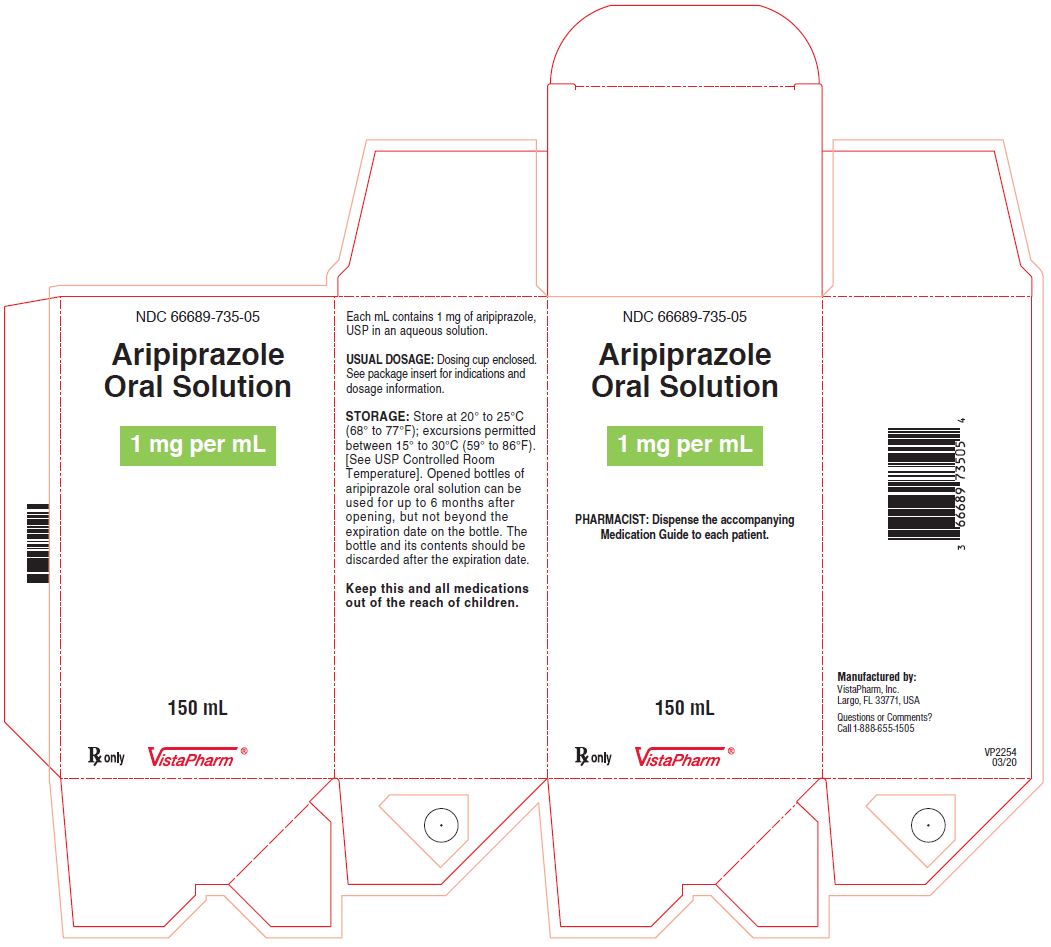
PRINCIPAL DISPLAY PANEL
NDC 66689-735-05
Aripiprazole
Oral Solution
1 mg per mL
PHARMACIST: Dispense the accompanying
Medication Guide to each patient
150 mL
Rx Only
VistaPharm®